- Science
- White Papers
- Oral TRT+ Protocol
- Abstract
- Background
- Treatment
- Statistical Analysis
- Results
- Discussion
- References
- Supplementary Material
- PHQ-4 questionnaire (Supplementary Material 1)
- qADAM questionnaire (Supplementary Material 2)
- Distribution of Treatments
- Post Treatment LH and FSH
- Pilot B Distribution
- BF+4 Distribution
- B+1 Distribution
- B+4 Distribution
- B+4 Post Treatment Distribution
Oral Testosterone and Enclomiphene Protocol
Oral Testosterone and Enclomiphene: A Novel, Non-Suppressive Strategy for Enhancing Testosterone in Hypogonadal and Eugonadal Men
S. Cameron Sepah, Ph.D.1,2; Gabriel Alizaidy, M.D., M.S.1; Hunter Henry, M.S.1; Nick Boerger, M.S.1
1. Maximus, Los Angeles, CA, United States
2. University of California, San Francisco, Department of Psychiatry, San Francisco, CA, United States
Corresponding Author:
S. Cameron Sepah, Ph.D.
Email: cam@maximustribe.com
Abstract
79 men classified as hypogonadal or eugonadal
47 referenced studies
Purpose
Male hypogonadism, characterized by decreased functionality of the testicles and evidenced by low testosterone levels, requires treatment strategies tailored to the specific type of hypogonadism. Primary hypogonadism, resulting from direct testicular impairment, is typically addressed with testosterone replacement therapy (TRT). In contrast, secondary hypogonadism, stemming from dysfunction in the hypothalamic-pituitary axis, often necessitates alternative treatments aimed at preserving fertility markers. These include Selective Estrogen Receptor Modulators (SERMs), Aromatase Inhibitors, and human chorionic gonadotropin (hCG). Although all treatment options can be effective, TRT uniquely offers dose-dependent benefits, which are not as achievable with alternatives due to their inability to bypass the body's natural testosterone production controls. However, the choice of TRT, particularly for individuals with secondary hypogonadism, involves a trade-off between maximizing treatment efficacy and preserving fertility markers. Our study addresses this dilemma by exploring a novel therapeutic approach, dubbed 'Oral TRT Plus,' which combines two oral medications: oral native testosterone and enclomiphene citrate. This approach aims to provide the dose-dependent benefits of exogenous testosterone while also preserving fertility markers.
Materials and Methods
79 male patients, encompassing both hypogonadal and eugonadal men, participated in a sequence of four distinct experiments. These experiments were designed to determine the ideal dosing, assess the necessity of fat intake for enhanced drug absorption, and identify the most effective timing for blood tests to capture peak testosterone levels. Measurements of various blood markers, including total testosterone, free testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and sex hormone-binding globulin (SHBG), were obtained using both at-home lab testing kits and in-person venipuncture. In addition, participants completed subjective questionnaires—the quantitative Androgen Deficiency in the Aging Male (qADAM) and the Patient Health Questionnaire-4 (PHQ-4)—to evaluate subjective improvements in hypogonadal symptoms during the treatment.
Results
With optimized dosing, total testosterone levels in both eugonadal and hypogonadal men increased fourfold and free testosterone fivefold (both p < 0.025), surpassing the 99th percentile for both measurements in all participants. Meanwhile, LH and FSH levels remained within normal ranges across all participants (p < 0.025), demonstrating the treatment's ability to preserve fertility markers irrespective of the increased testosterone dosing. Estrogen levels significantly decreased but stayed within normal limits. Subjective health measures improved notably; over 70% of participants reported enhanced energy, strength/endurance, improved work performance, and quality of life according to qADAM scores, with significant reductions in anxiety and depression symptoms evidenced by 97% of participants reporting normal function (both p < 0.001).
Conclusion
Both eugonadal and hypogonadal men utilizing the “Oral TRT Plus Protocol” demonstrated a fivefold increase in free testosterone levels on average, while simultaneously mitigating endogenous suppression and preserving fertility markers. This protocol elicited significant improvements across various subjective health metrics, including quality of life, energy, strength, mood, libido, and work performance, alongside a marked decrease in anxiety and depression symptoms. Furthermore, the protocol effectively regulated estrogen levels within normal physiological ranges, eliminating the necessity for adjunctive medications. The “Oral TRT Plus Protocol” aligns with the evolving landscape of telemedicine by demonstrating how advanced hormonal treatment regimens can be effectively administered and monitored remotely, offering an innovative, non-invasive strategy for enhancing men's mental, physical, and hormonal health while preserving fertility markers.
Background

Testosterone is known to decline by 1-2% annually beyond the age of 30, often due to lifestyle and environmental factors.
From its secretion by fetal Leydig cells to differentiate male genitalia, to driving masculinization from puberty through adulthood, testosterone plays key roles in the growth, development and maintenance of various physiological functions that define the male sex.1 Its influence extends beyond the visible traits of masculinity, impacting bone density, muscle strength, fat distribution, and the regulation of mood and energy levels.2 Moreover, testosterone is crucial for metabolic health, libido, and sperm production, emphasizing the necessity for optimal levels across all stages of male development and aging.3,4,5 Testosterone is known to decline by 1-2% annually beyond the age of 30, often due to lifestyle and environmental factors.6 The quintessential presentation of low testosterone includes decreased muscle mass, increased body fat, reduced energy levels, decreased libido and erectile function, and potential mood disorders.7
Male hypogonadism is defined as decreased functional activity of the testes, as characterized by a reduction in testosterone levels.8

There are 2 main forms of male hypogonadism
These treatment modalities are ineffective in the case of primary hypogonadism, where signaling pathways are intact. Therefore, traditional testosterone replacement therapy (TRT), including injections, creams, and patches, has become the mainstay treatment in the case of primary hypogonadism. In all cases of male hypogonadism, clinical outcomes are dependent on improving levels of testosterone.10 TRT can also be used to treat secondary hypogonadism; however, due to its impact on gonadotropin secretion through negative feedback mechanisms, it also decreases testicular output of testosterone and other aspects of fertility.11 The subsequent decreases in testicular function and fertility markers are so profound that TRT is strictly contraindicated for the treatment of male infertility by the European Association of Urology.12 Maintenance of fertility is the determinant factor in choosing treatment options for secondary hypogonadism by the American Urology Association.9
Negative feedback mechanisms from excess estradiol (E2), testosterone (T), and dihydrotestosterone (DHT), downregulate the secretion of LH from the pituitary gland.13 SERMs, such as enclomiphene, inhibit this negative feedback via selective estrogen receptor blocking in the hypothalamus and pituitary gland. This blockade promotes testosterone production from the Leydig cells via LH secretion, and improves spermatogenesis by stimulating Sertoli cells via FSH secretion.14 While the increase in LH promotes an increase in testosterone, the negative feedback from subsequent increases in T and DHT, combined with variations in LH receptor sensitivity, enzymatic activity, and substrate availability, limit the output of testosterone by the testicles.15
Treatments for hypogonadism that focus on maintaining fertility markers, while effective, are inherently limited in the amount of testosterone that can be produced. TRT, by providing exogenous testosterone, bypasses the necessity for Leydig cell contribution to serum testosterone and allows for dose dependent increases in testosterone beyond what the testicles can produce.

However, the introduction of exogenous testosterone to the body drastically reduces spermatogenesis resulting in decreased fertility.16
Gonadotropin secretion is vital for the regulation of spermatogenesis, the biological process by which sperm cells are produced in the testes of males. FSH directly stimulates Sertoli Cells in the testicles to nurture developing sperm cells and has traditionally been used as a proxy marker for spermatogenic status.17 LH stimulates Leydig cells in the testicles to produce testosterone local to the testicles, or intratesticular testosterone (ITT). In combination with FSH, ITT is necessary to induce optimal spermatogenesis.18 This requirement is unique to ITT as exogenous testosterone combined with FSH cannot induce optimal spermatogenesis.
All forms of traditional TRT decrease FSH by up to 86.3% and LH up to 71.8%, on average.19 In healthy eugonadal men subjected to TRT, ITT levels decreased by 94%.20 Recovery from this suppression is not a certainty after cessation of exogenous testosterone, with some men taking up to 24 months to recover normal sperm count.21 In order to combat this suppression, TRT has been paired with Human Chorionic Gonadotropin (HCG), an LH mimetic, that can signal the testicles to produce more ITT.20 Adding recombinant FSH (rFSH) improves semen parameters by stimulating the Sertoli cell component of spermatogenesis.22 The drawbacks of this combination treatment go beyond the inconvenience of injecting two ancillary drugs to preserve spermatogenesis. Gonadotropin therapy can cause a steep upregulation of aromatase, leading to estrogenic side effects such as gynecomastia, and an acute and sustained increase in prolactin.23,24
Short acting forms of testosterone, like oral T, are less suppressive than long acting forms of testosterone, and so a combination of oral T and a SERM, Tamoxifen, was hypothesized to provide the benefits of exogenous testosterone while utilizing the negative feedback inhibition of Tamoxifen to promote and maintain gonadotropin secretion.19,25 A low dose of oral testosterone undecanoate, dosed at 40 mg three times daily, combined with tamoxifen citrate, dosed at 10 mg twice daily, showed no adverse effects on pituitary or Leydig cell activity, and showed improvements in semen parameters compared to the tamoxifen only group.25

Previous studies have revealed 3 important outcomes regarding the hormonal modulation in response to exogenous T and SERM administration, and served as the basis for our experiments.
- It was observed that the suppressive effects of low-dose testosterone on the hypothalamic-pituitary-gonadal axis could be effectively mitigated through the administration of SERMs.
- The combination treatment of low-dose testosterone and a SERM was associated with maintained, and in some instances, elevated gonadotropin levels.
- A positive correlation between higher levels of LH and FSH and the improvement of semen parameters was identified with the combination treatment.
The suppressive nature of exogenous T can also be determined by the dose administered. A study measuring the effects on LH and FSH from different dosing of injected testosterone enanthate showcased a decrease in LH and FSH at day 14 by 92% and 94% with 500 mg, and a decrease in LH and FSH at day 14 by 45% and 38% with 125 mg.26 The study combining oral T and Tamoxifen used a low dose of testosterone that yielded basal T levels of 242 ng/dL, which is well below the typical 300 ng/dl threshold for hypogonadism. The low dose of oral T, combined with the outcome of low T levels, could potentially confound the results. This is because the dose and effect of the oral T might be insufficient to trigger the negative feedback mechanism on the Hypothalamic-Pituitary-Gonadal (HPG) axis, a phenomenon observed when SERMs are used in conjunction with longer-acting and higher-dosed forms of TRT, and thus leading to the recommendation to stop TRT before pursuing treatment options to restart the HPG axis.27

The purpose of this study was to determine whether a SERM, combined with higher doses of oral testosterone, can significantly improve serum T levels and T associated symptoms, while prioritizing the maintenance, or even improvement, of fertility markers.
Treatment
In this study, participants were administered a combination therapy involving oral testosterone in the form of native testosterone lipid bilayer tablets and enclomiphene citrate. The dosing of these medications varied across the four experiments conducted, reflecting adjustments based on specific objectives and findings at each stage. In general, the initial dosing was informed by prepublished data, with oral native testosterone prescribed at varying dosages up to 800 mg per day, administered in a single bolus dose. The enclomiphene dosage also varied, initially set at 3.125 mg or 6.25 mg, but adjusted in some cases based on its effectiveness in counteracting the suppressive effects of exogenous testosterone on LH and FSH levels.

Previous studies on oral testosterone indicated favorable absorption with 30 grams of fat.28
Therefore, participants were advised to consume at least 30 g of fat to aid in the absorption of the oral testosterone via lymphatics, although this requirement was also explored and tested within the different experiments.29
Participants receiving a combined treatment of oral testosterone and enclomiphene are expected to show a significant increase in total and free testosterone levels. This increase in testosterone may also lead to higher estrogen levels, through aromatization. This increase is understood to cause of gynecomastia and/or breast pain that accompanies testosterone replacement therapy.30 Additionally, based on existing literature, androgen therapy, such as the administration of testosterone, has been shown to decrease Sex Hormone Binding Globulin (SHBG) levels.31 A reduction in SHBG is therefore expected as a result of the treatment which would then increase the amount of active, free testosterone.
Gonadotropins, secreted by the anterior pituitary gland, play a key role in testosterone production and fertility. LH is important as it serves as a measure of the signal to leydig cells in the testes to produce testosterone and a proxy for measuring endogenous testosterone production in the testes.32 FSH is a crucial indicator of male fertility, essential for the regulation of spermatogenesis via sertoli cells, thereby directly impacting male reproductive capability.32 While shorter acting forms of testosterone therapy suppress FSH and LH less than longer acting forms, both still cause a steep reduction from baseline levels.19 It is therefore understood that both long and short-acting testosterone therapy negatively impact fertility without ancillary treatment. Oral T is a short-acting testosterone therapy where T levels peak at 4-5 hours after administration and return to baseline at approximately 12 hours.33 Enclomiphene is a SERM that modulates estrogen receptors in the hypothalamus and pituitary gland to increase LH and FSH signaling, thus improving endogenous testosterone production and fertility.34 Through this mechanism, it is postulated to mitigate the suppressive effects of oral testosterone and therefore throughout the treatment process, the levels of LH and FSH are hypothesized to either remain consistent with baseline measurements or show improvement.

Pilot B
This experiment was designed to explore the effects of oral testosterone and enclomiphene on hormonal levels. The variability in testosterone dosing (ranging from 200 to 600 mg) was based on findings from pre-published trials, which suggested that individuals with higher baseline levels of free and total testosterone require lower doses to achieve desired increases in testosterone levels. For this pilot study, the testing was conducted 4 hours after administration, aligning with literature on oral testosterone undecanoate, which indicated peak concentrations around this time.28 Participants were instructed to consume 30 g of fat alongside the dose, a protocol derived from studies on oral testosterone undecanoate, where fat intake was shown to improve absorption.

BF+4
BF+4 was designed to test the absorption and efficacy of the treatment under fasted conditions, based on a trial demonstrating that oral native testosterone tablets absorbed well without the need for dietary fat.35 In this experiment, a standardized dose of 800 mg of oral testosterone was given to all participants.

B+1 and B+4
B+1 aimed to determine if the peak testosterone level occurred at 1 hour post-administration, a timing observed in trials with oral native testosterone tablets.35 During both of these experiments, participants were encouraged to consume a minimum of 30 g of fat along with the medication. B+4, conducted on the same day, was designed to compare whether the peak level was more pronounced at 1 hour or at 4 hours post-administration. This comparison was crucial for understanding the pharmacokinetic profile of the treatment regimen in a food-influenced state, providing valuable insights into the timing and absorption of the drug under these specific conditions.
Statistical Analysis

We hypothesized that all experimental groups would demonstrate baseline or elevated LH and FSH levels in post-treatment blood assessments, regardless of the increase in testosterone.
In our study, we employed the Shapiro-Wilk test to assess the variability in distribution characteristics across different parameters. This analysis indicated that while baseline total testosterone (Total T) and baseline free testosterone (Free T) were normally distributed, other parameters such as luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and sex hormone-binding globulin (SHBG) deviated from a normal distribution. In light of these findings, we chose the Wilcoxon signed-rank test, a non-parametric method, for analyzing changes from baseline to post-treatment across all variables.
Our decision to use the Wilcoxon test was driven by the need for a consistent methodological approach capable of handling both normally and non-normally distributed data. This was particularly relevant given the small sample sizes and the presence of outliers in certain variables. Moreover, we tailored the application of the Wilcoxon test to align with our hypothesized direction of change for each variable. For variables where an increase was anticipated, such as Total T, Free T, and E2, and where maintenance or an increase was expected, as in levels of LH and FSH, we used a right-tailed Wilcoxon test. Conversely, for variables where a decrease was hypothesized, namely SHBG, we implemented a left-tailed Wilcoxon test. An alpha level of 0.025 was universally chosen to enhance the robustness of our findings.
Building on this approach, we hypothesized that all experimental groups would demonstrate baseline or elevated LH and FSH levels in post-treatment blood assessments, regardless of the increase in testosterone. The Shapiro-Wilk test results for the aggregated LH and FSH values did not confirm a normal distribution, necessitating the use of a non-parametric test. Accordingly, we employed a right-tailed Wilcoxon Signed-Rank Test, which was aligned with our hypothesis of detecting an increase in LH and FSH levels.
Upon analyzing the results, we observed high p-values, close to 1, in the B+1 and B+4 right-tailed Wilcoxon tests, along with a noticeable decrease in estrogen levels. These findings prompted us to reevaluate our approach for estrogen. We then decided to conduct a left-tailed Wilcoxon test to determine if the observed decrease in estrogen was statistically significant.
We evaluated treatment efficacy by measuring both the overall average change in values before and after treatment and the average of individual relative changes. This latter approach emphasizes the significance of capturing each participant's unique response to the treatment. By calculating the relative change for each individual—essentially, their percentage improvement—and then averaging these figures, we incorporate the vast array of personal factors, such as age and dietary fat intake, that can potentially affect treatment outcomes.
Results

Pilot B
With varying testosterone doses between 200-600 mg, revealed that these doses were less effective at consistently elevating testosterone levels compared to the standardized 800 mg doses (Table 7). For example, in Pilot B, the average increase in total testosterone was 414.6 ng/dL, showing an average relative change of 169.4%, whereas the BF+4 experiment with an 800 mg dose under suboptimal fasting conditions showed a more significant increase of 661.6 ng/dL (262.9% average relative change).

BF+4
Conducted under fasting conditions, the testosterone levels might have been artificially elevated due to the protocol instructing participants to eat only 1 hour after dosing (Table 8). Participants who reported fasting for over 3 hours post-dosing had testosterone levels below 600 ng/dL, but greater than 400 ng/dL, indicating the substantial impact of fasting and the timing of fat consumption on treatment efficacy.

B+1
Measuring testosterone levels 1 hour post-dosing, showed lower increases in testosterone levels compared to the 4-hour tests (Table 9). Total testosterone increased by 415.4 ng/dL (182.1% relative change), and free testosterone by 135.5 pg/mL (240.3% relative change), suggesting that 1 hour post-administration is less effective for measuring peak testosterone levels.

B+4
It involved administering the treatment with at least 30 g of fat and testing 4 hours later, demonstrated the highest increases in both total and free testosterone (Table 10). The increase in total testosterone was 865.5 ng/dL (307.6% relative change), and free testosterone increased by 295 pg/mL (470.1% relative change), indicating that the 4-hour post-dosing period is optimal for assessing peak testosterone levels. The average result of total testosterone was 1327.6 ng/dL with an average free testosterone of 383 pg/mL. It is important to note that while these levels may seem elevated and supraphysiological, the increase is temporary and mimics a spike similar to how natural diurnal testosterone secretions function.36

With optimized dosing, both total and free testosterone levels showcased in the 99th percentile when compared to 19-40 year old never-smoking, lean men without aging-associated comorbidities.
Regardless of dosing, fat intake, and time when testosterone was measured, when compared to 19-40 year old never-smoking, lean men without aging-associated comorbidities, both total and free testosterone showcased at the 92nd percentile or higher, with the optimized dosing showing both total and free testosterone levels in the 99th percentile (Table 6).37 This population serves as a better comparison because it does not include comorbidities, such as high BMI and aging, which could otherwise normalize data against a less healthy baseline.
A critical aspect of this study was the analysis of LH and FSH levels. Individual experiments did not demonstrate statistically significant increases in LH and FSH due to enclomiphene dosing being optimized with each experiment. However, when LH and FSH data were aggregated and compared from baseline to post-treatment, a statistically significant elevation was observed (Tables 1,3,4). This finding is of particular interest as testosterone replacement therapy commonly results in the suppression of these hormones. The treatment protocol employed in this study effectively mitigated the suppression of LH and FSH, which is consistent with the primary aim of augmenting testosterone levels while sustaining LH and FSH.
Furthermore, it was observed that 100% of participants, following treatment, exhibited LH and FSH levels within clinically normal ranges, with LH exceeding 1.8 IU/L and FSH above 1.5 IU/L.38,39,40 This consistent maintenance of LH and FSH within normal physiological limits across the participant cohort indicates the universal efficacy of the treatment in mitigating hormonal suppression.
Contrary to our initial expectations of an increase in estrogen levels, the B+1 and B+4 experiments showed a significant decrease, with reductions of -18.5% and -7.1% respectively, confirmed as statistically significant through left-tailed Wilcoxon tests (Table 11).
In the B+4 experiment, a secondary analysis focused on identifying patients who met established testosterone thresholds, which are categorized as 'conventional average' and 'optimal' based on public perception and commonly recognized whole numbers (Table 2). The 'conventional average' category, a layman's equivalent to what might be clinically described as 'normal', is set at 500 ng/dL for total testosterone and 100 pg/mL for free testosterone. These levels are widely acknowledged as typical or average in the general population, though not necessarily derived from rigorous scientific consensus. Conversely, the 'optimal' category, representing thresholds of 1000 ng/dL for total testosterone and 200 pg/mL for free testosterone, is seen as indicative of superior physiological conditions. This analysis, by adopting these widely recognized thresholds, offers insights that align with the general public's understanding of testosterone levels.
Significant hormone level changes were seen across the interquartile ranges in the B+4 experiment (Table 5). Total testosterone levels showed a relative increase of 1.88 times at the 25th percentile, 3.59 times at the 50th percentile, and 4.65 times at the 75th percentile. Similarly, free testosterone levels increased 3.13 times at the 25th percentile, 4.35 times at the 50th percentile, and 6.8 times at the 75th percentile. In contrast, estrogen levels exhibited changes of 0.68 times at the 25th percentile, 0.85 times at the 50th percentile, and 1.0 times at the 75th percentile.
Participants were directed to take an in depth questionnaire at the beginning and completion of the study that included their current medications, relevant medical history, a quantitative questionnaire used to screen for androgen deficiency, and questions from the Patient Health Questionnaire-4 (PHQ4) (Supplementary Material 1).41 The quantitative Androgen Deficiency in the Aging Male (qADAM), uses graded responses to 10 questions concerning symptoms of androgen deficiency (Supplementary Material 2).42
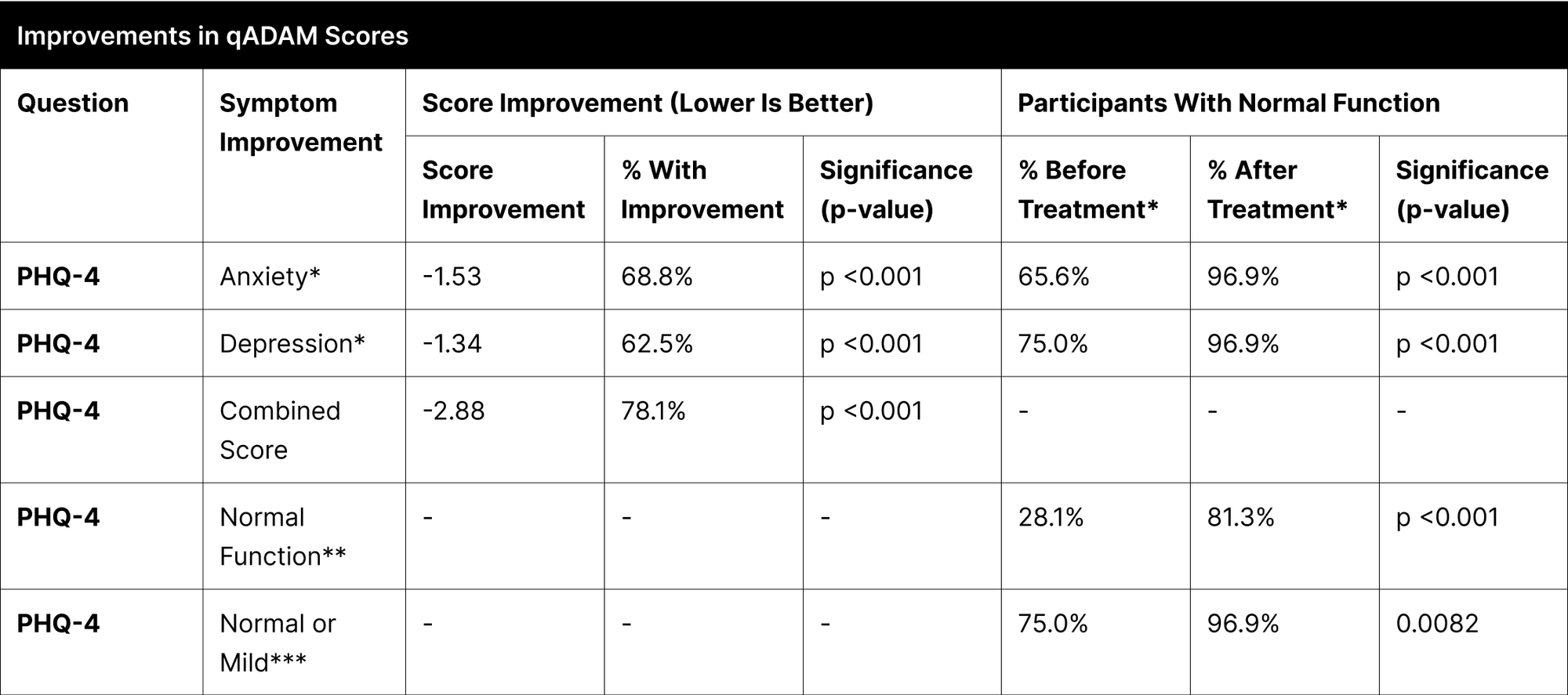
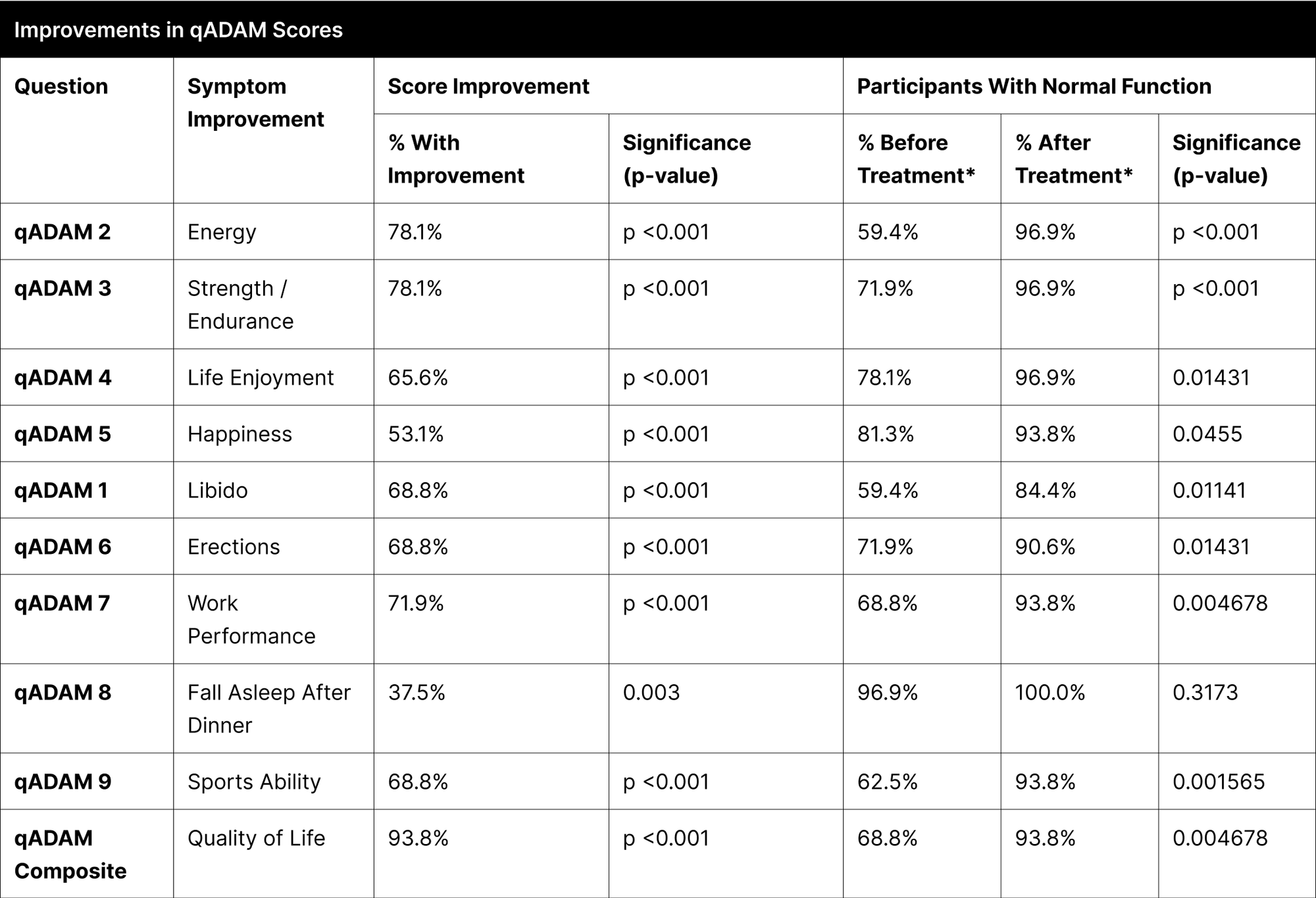
A significant proportion of participants experienced enhancements in their reported symptoms, with the most notable improvements observed in energy, strength/endurance, and the qADAM composite score indicating overall quality of life, each showing over 70% improvement (Table 12). Other categories like life enjoyment, happiness, libido, erections, and sports ability also demonstrated considerable improvements, ranging between 53% to 69%.
In the specific case of "Fall Asleep After Dinner," the improvement was not statistically significant due to the high pretreatment normal function rate of 96.9%, which further increased to 100% post-treatment. Treatment efficacy was substantial and widespread, as indicated by over 93.8% of participants achieving normal function post-treatment in most categories. The categories of libido and erections, although not reaching the same levels, also showed significant improvements, with 84.4% and 90.6% of participants achieving normal function, respectively.
For Anxiety, 68.8% of participants reported improvement, with the percentage of those achieving normal function increasing from 65.6% to 96.9%. In Depression, 62.5% of participants improved, and the proportion reaching normal function rose from 75.0% to 96.9%.
In the PHQ-4 combined score, which encompasses both anxiety and depression, 78.1% of participants exhibited improvement (Table 13). The percentage of participants achieving normal function in this measure increased significantly from 28.1% to 81.3%. Additionally, those with normal or mild function on the PHQ-4 combined score improved from 75.0% to 96.9%.
Table 1
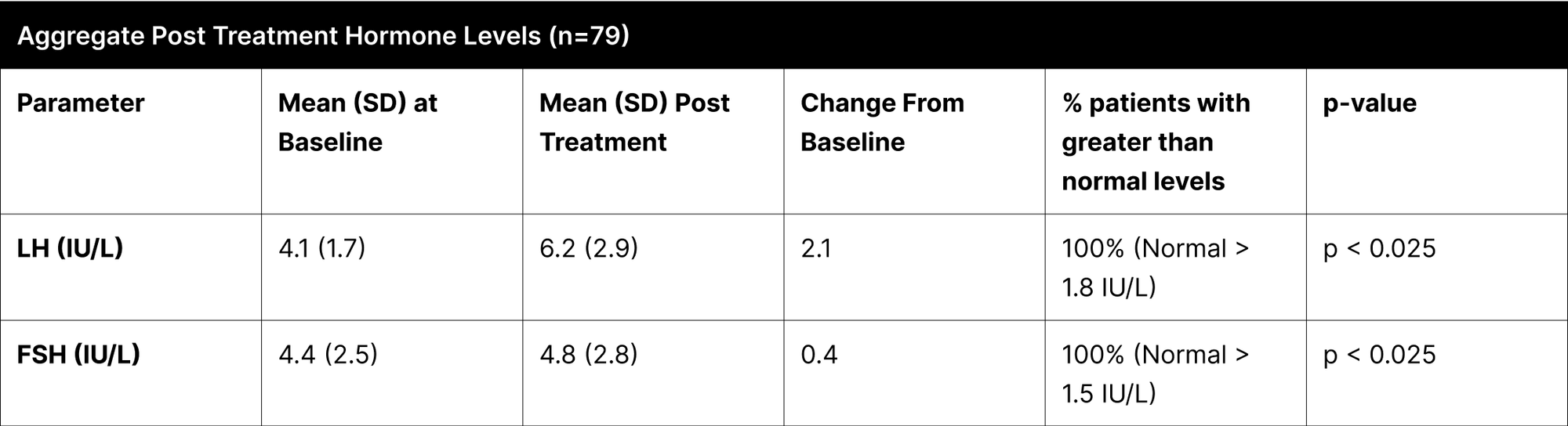
Table 2
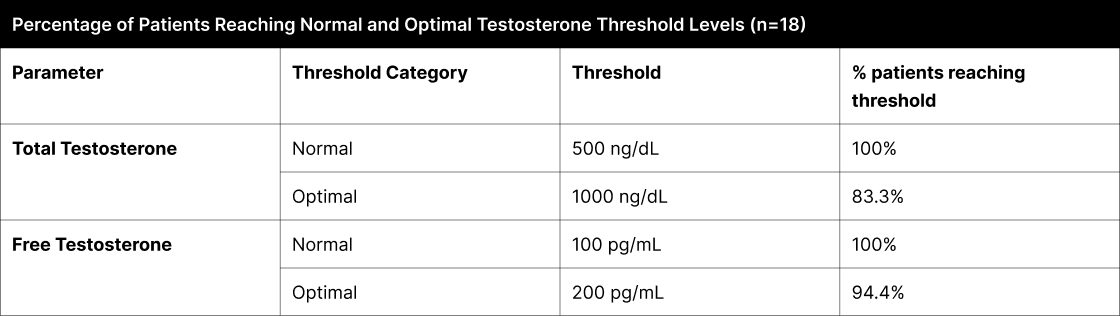
Table 3
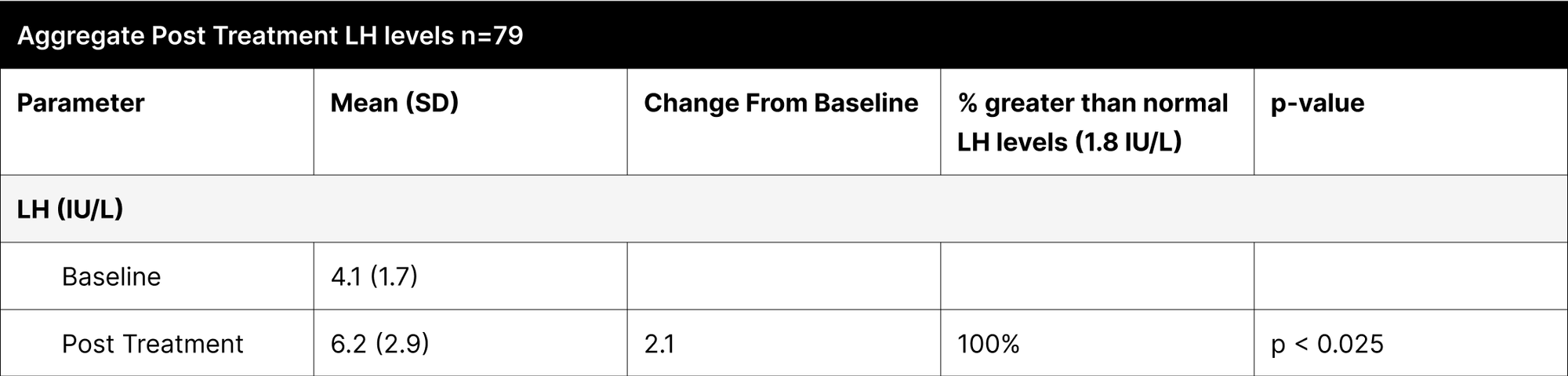
Table 4
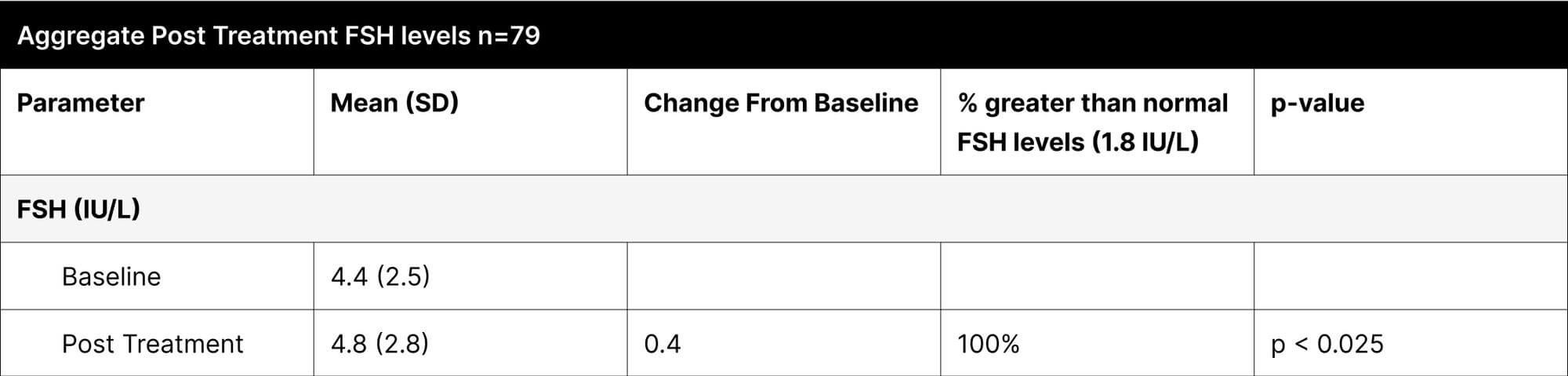
Table 5
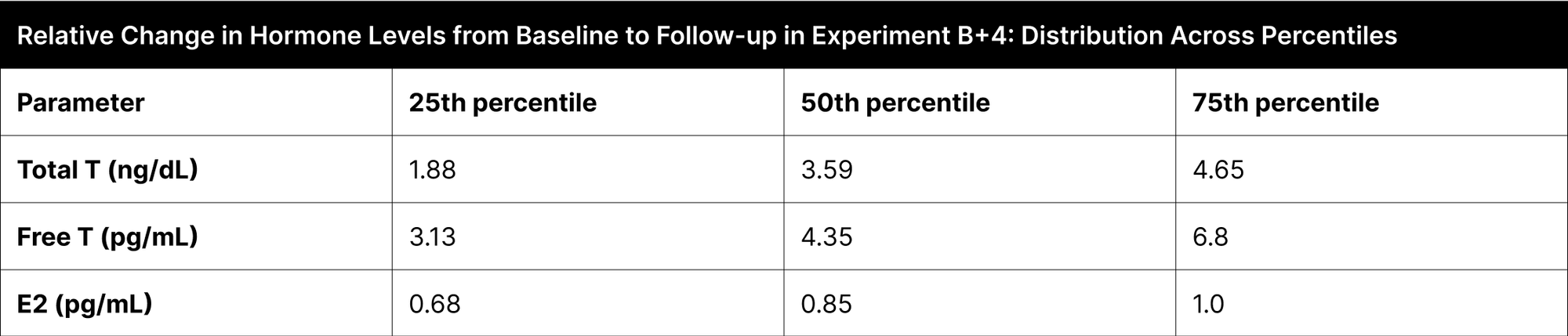
Table 6
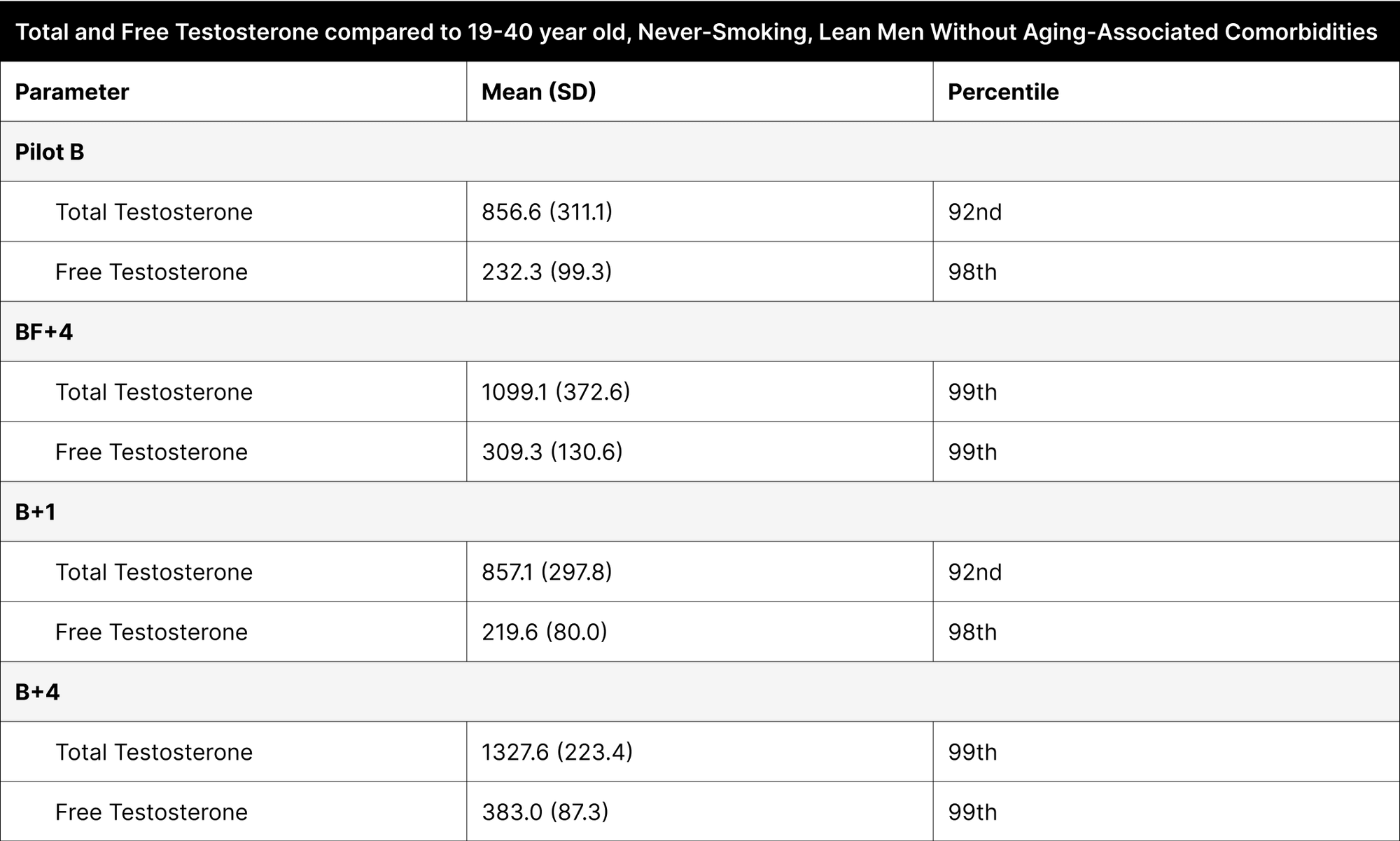
Table 7
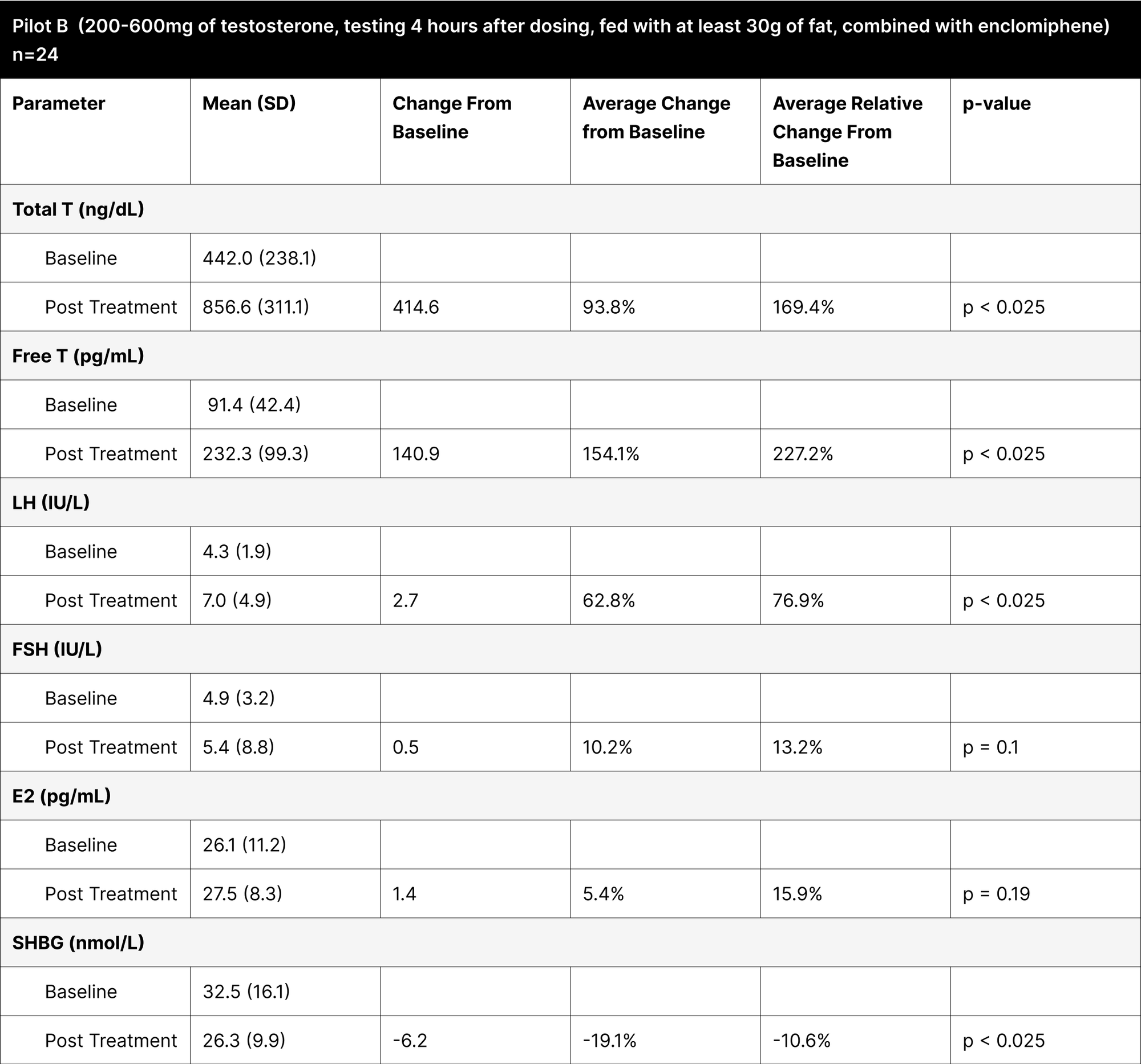
Table 8
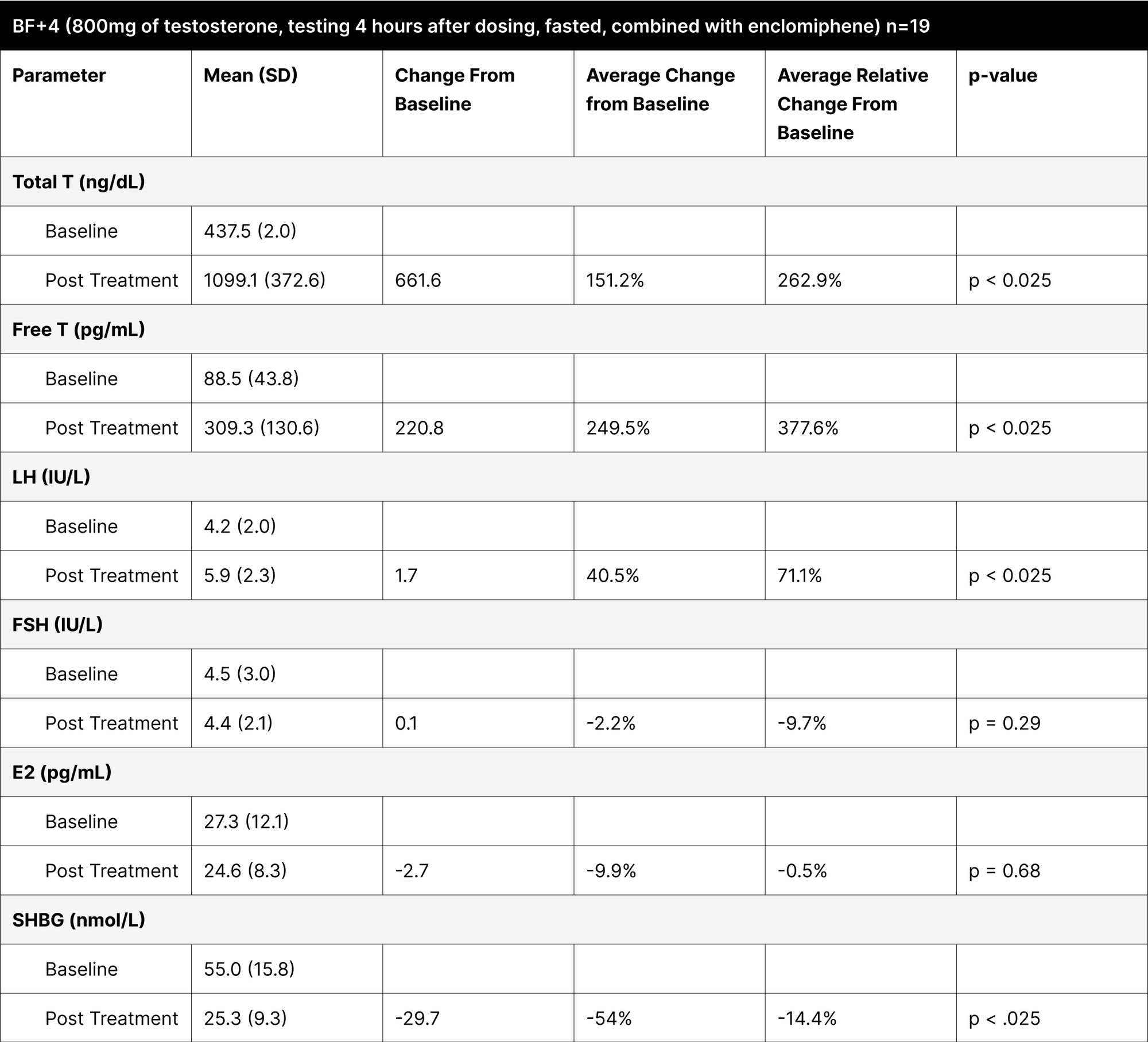
Table 9
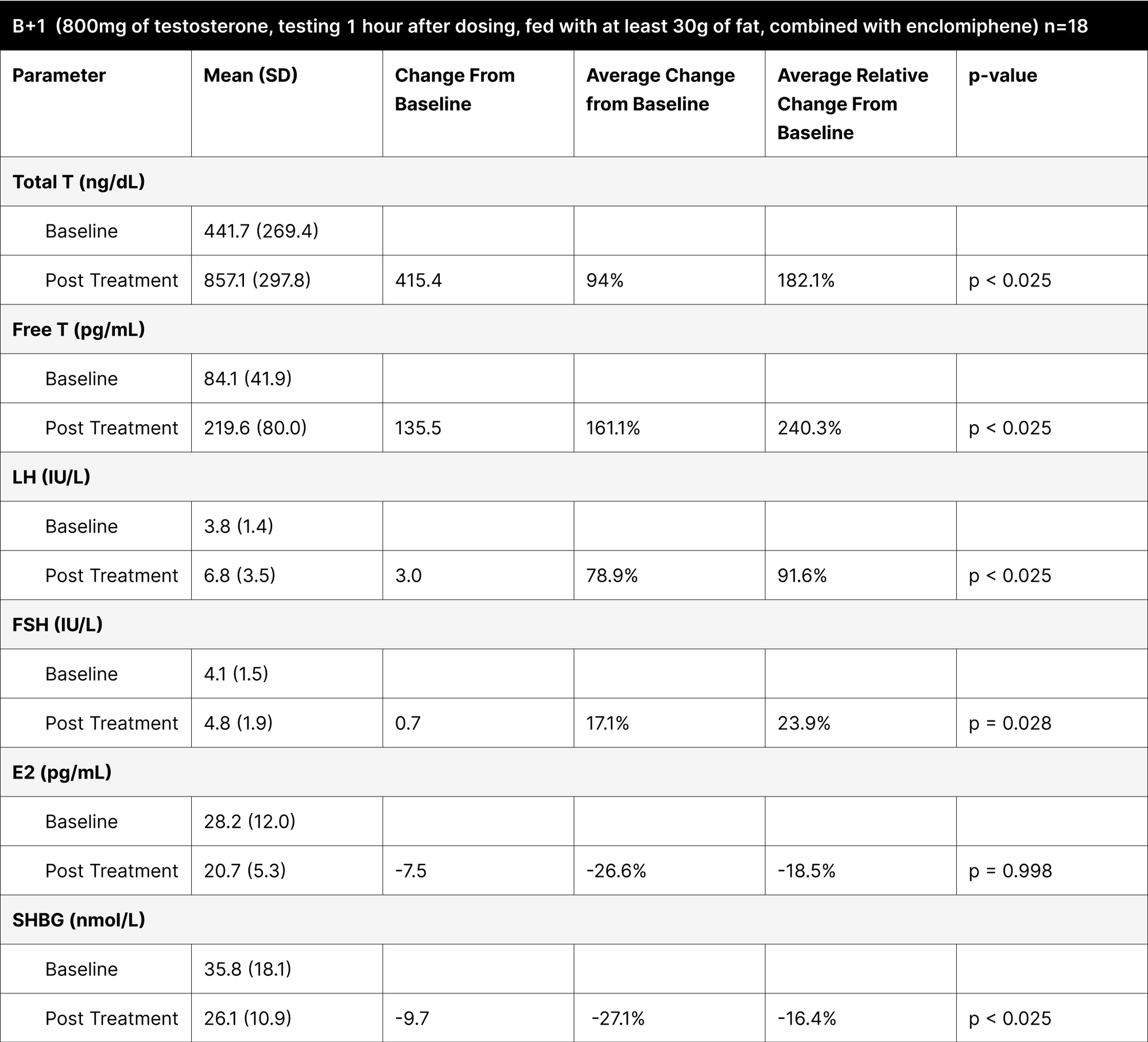
Table 10
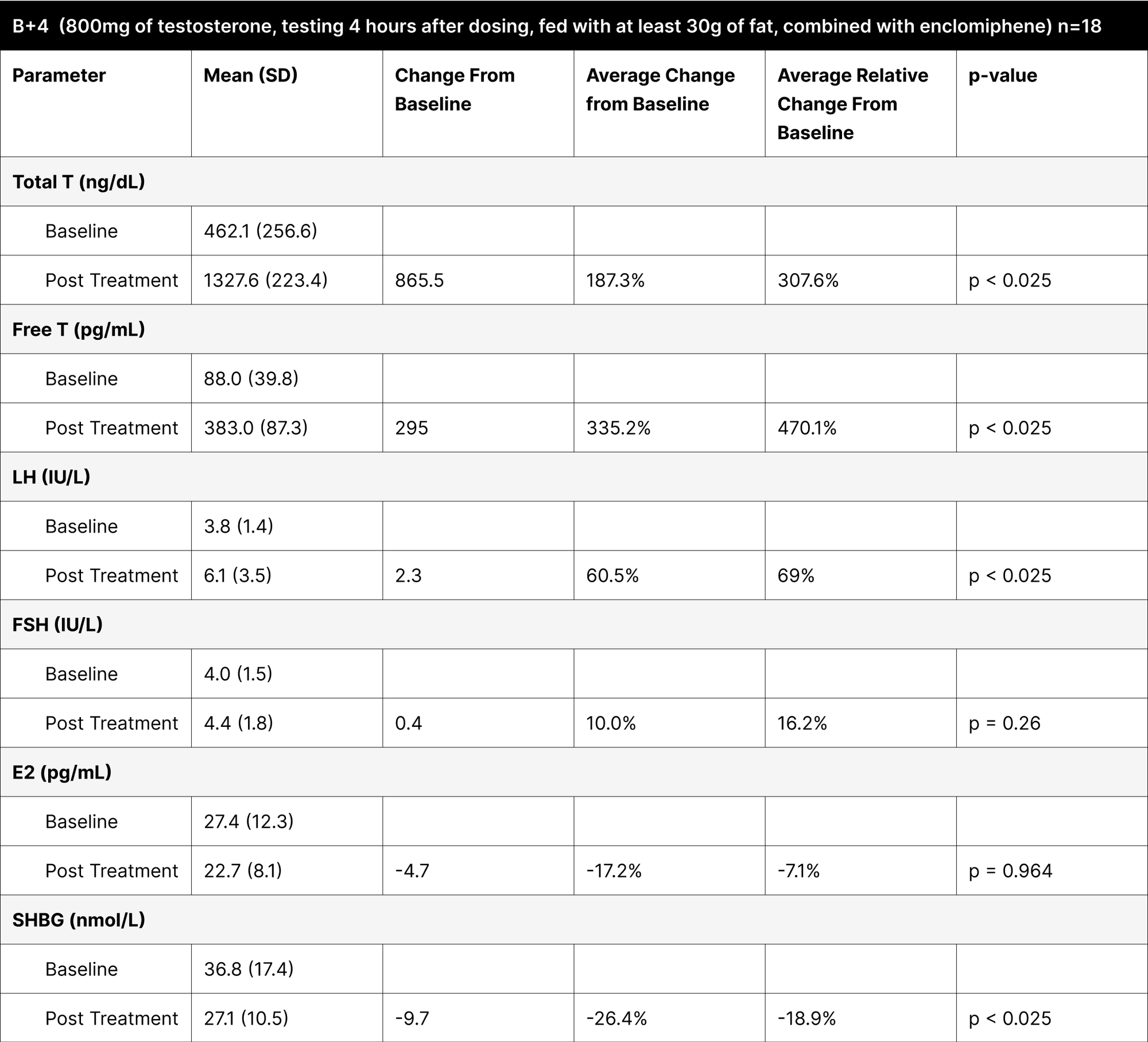
Table 11
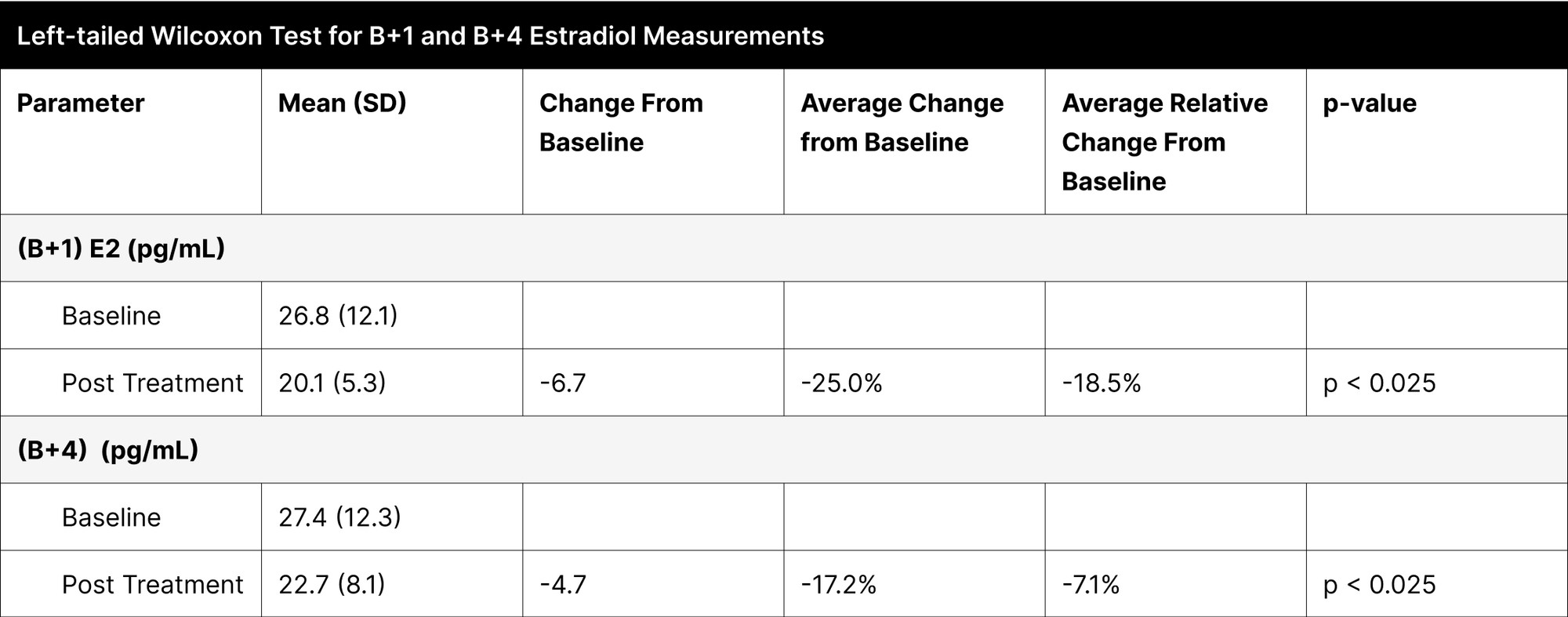
Table 12
* Normal function is defined as a score of at least 3 for individual questions and average of 30 for the composite.42
Discussion
Building on the findings from our study, these results represent a significant scientific breakthrough and a previously undocumented ability to effectively mitigate suppression from testosterone therapy without the use of injectable modalities. The substantial increases in testosterone levels, particularly highlighted in the B+4 experiment, demonstrate the efficacy of this combined treatment approach. This balance is vital as it ensures that while testosterone levels are significantly enhanced, the treatment also protects fertility, thereby allowing secondary hypogonadal and eugonadal men to utilize this treatment without fearing negative outcomes from endogenous suppression.

This treatment not only significantly increases testosterone levels but also maintains fertility markers, enabling men with secondary hypogonadism and eugonadal conditions to use this therapy without worrying about adverse effects on their natural hormone production.
Additionally, an observed reduction in estrogen levels further underscores the uniqueness of this approach. By lowering estrogen, the treatment circumvents complications commonly associated with high estrogen levels during TRT, such as gynecomastia, mood swings, and water weight gain. This aspect of the treatment not only adds to its efficacy but also contributes to a more comprehensive management of hormonal imbalances without the need of added ancillary treatments.
Commonly noted benefits of testosterone therapy include improvements in energy, libido, muscle mass, well-being, fat reduction, and social energy, as evidenced by open ended surveys of men’s experiences on testosterone therapy.43 Improvements in these categories were expected in our study, but the overwhelming and ubiquitous nature of the improvement was not, as showcased by 93.8% of participants showing improvement in the overall composite qADAM score. While testosterone is not a psychoactive hormone in the classical sense, it does have some effects on the brain and behavior, as indicated by the improvements in happiness, anxiety, and depression. Testosterone is postulated to have anxiolytic properties through modifications in reward processing and stress resilience.44,45 Low testosterone has been associated with depression and reduction in depressive symptoms has been seen across multiple studies after starting testosterone treatment.46,47

Collectively, these 4 benefits forge
a new pathway in men’s health optimization
- The enhancement of testosterone levels
- The near-universal improvement in quality of life
- The preservation of LH and FSH levels
- The reduction in estrogen
This triad approach broadens the scope of treatment possibilities, extending its applicability beyond individuals with primary hypogonadism. It empowers healthcare practitioners to adopt a more comprehensive and patient-centered strategy in managing hormonal disorders, focusing on symptom resolution rather than solely numerical hormone level adjustments.
References
- Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation��†. Biology of Reproduction. 2018;99(1):101-111. doi:10.1093/biolre/ioy059
- Emmelot-Vonk MH, Verhaar HJJ, Pour HRN, et al. Effect of Testosterone Supplementation on Functional Mobility, Cognition, and Other Parameters in Older Men. JAMA. 2008;299(1). doi:10.1001/jama.2007.51
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nature Reviews Endocrinology. 2009;5(12):673-681. doi:10.1038/nrendo.2009.212
- Rizk P, Kohn TP, Pastuszak AW, Khera M. Testosterone therapy improves erectile function and libido in hypogonadal men. Current Opinion in Urology. 2017;27(6):511-515. doi:10.1097/mou.0000000000000442
- McLachlan RI, O’Donnell L, Meachem SJ, et al. Hormonal regulation of spermatogenesis in primates and man: Insights for development of the male hormonal contraceptive. Andrology. 2002;23(2):149-162. doi:10.1002/j.1939-4640.2002.tb02607.x
- Feldman HA, Longcope C, Derby CA, et al. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. The Journal of Clinical Endocrinology & Metabolism. 2002;87(2):589-598. doi:10.1210/jcem.87.2.8201
- Cohen JY, Nassau DE, Patel P, Ramasamy R. Low Testosterone in Adolescents & Young Adults. Frontiers in Endocrinology. 2020;10. doi:10.3389/fendo.2019.00916
- Carnegie C. Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol. 2004;6 Suppl 6(Suppl 6):S3-S8.
- Testosterone Deficiency Guideline - American Urological Association. https://www.auanet.org/guidelines-and-quality/guidelines/testosterone-deficiency-guideline
- Ponce OJ, Spencer‐Bonilla G, Álvarez-Villalobos NA, et al. The Efficacy and Adverse Events of Testosterone Replacement Therapy in Hypogonadal Men: A Systematic Review and Meta-Analysis of Randomized, Placebo-Controlled Trials. The Journal of Clinical Endocrinology & Metabolism. 2018;103(5):1745-1754. doi:10.1210/jc.2018-00404
- Sheckter CB, Matsumoto AM, Bremner WJ. Testosterone Administration Inhibits Gonadotropin Secretion by an Effect Directly on the Human Pituitary*. The Journal of Clinical Endocrinology & Metabolism. 1989;68(2):397-401. doi:10.1210/jcem-68-2-397
- MALE HYPOGONADISM - Uroweb. Uroweb - European Association of Urology. https://uroweb.org/guidelines/sexual-and-reproductive-health/chapter/male-hypogonadism
- Hohl A, ed. Testosterone: From Basic to Clinical Aspects. 2nd ed. Cham, Switzerland: Springer; 2023. doi:10.1007/978-3-031-31501-5.
- Kaminetsky J, Werner M, Fontenot G, Wiehle RD. Oral Enclomiphene Citrate Stimulates the Endogenous Production of Testosterone and Sperm Counts in Men with Low Testosterone: Comparison with Testosterone Gel. The Journal of Sexual Medicine. 2013;10(6):1628-1635. doi:10.1111/jsm.12116
- Midzak A, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Molecular and Cellular Endocrinology. 2009;299(1):23-31. doi:10.1016/j.mce.2008.07.016
- Desai A, Yassin M, Cayetano A, Tharakan T, Jayasena CN, Minhas S. Understanding and managing the suppression of spermatogenesis caused by testosterone replacement therapy (TRT) and anabolic-androgenic steroids (AAS). Ther Adv Urol. 2022;14:17562872221105017. Published 2022 Jun 26. doi:10.1177/17562872221105017
- Santi D, Crépieux P, Reiter É, et al. Follicle-Stimulating Hormone (FSH) Action on Spermatogenesis: A Focus on Physiological and Therapeutic Roles. Journal of Clinical Medicine. 2020;9(4):1014. doi:10.3390/jcm9041014
- Schaison G, Young J, Pholsena M, Nahoul K, Couzinet B. Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. The Journal of Clinical Endocrinology & Metabolism. 1993;77(6):1545-1549. doi:10.1210/jcem.77.6.8263139
- Masterson TA, Turner D, Vo D, et al. The Effect of Longer-Acting vs Shorter-Acting Testosterone Therapy on Follicle Stimulating Hormone and Luteinizing Hormone. Sexual Medicine Reviews. 2021;9(1):143-148. doi:10.1016/j.sxmr.2020.07.006
- Lee JA, Ramasamy R. Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men. Translational Andrology and Urology. 2018;7(S3):S348-S352. doi:10.21037/tau.2018.04.11
- Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. The Lancet. 2006;367(9520):1412-1420. doi:10.1016/s0140-6736(06)68614-5
- Hakky T, Shirazi H, Christensen MJ, Pastuszak A. 5 Continuous Testosterone Therapy with Recombinant FSH & HCG Improves Semen Parameters: A Pilot Study. The Journal of Sexual Medicine. 2022;19(Supplement_1):S3. doi:10.1016/j.jsxm.2022.01.016
- Boeri L, Capogrosso P, Salonia A. Gonadotropin Treatment for the Male Hypogonadotropic Hypogonadism. Current Pharmaceutical Design. 2021;27(24):2775-2783. doi:10.2174/1381612826666200523175806
- Martikainen H. RELATION OF hCG‐STIMULATED STEROIDOGENESIS TO SERUM FSH, LH AND PROLACTIN IN MAN. Clinical Endocrinology. 1981;15(3):283-289. doi:10.1111/j.1365-2265.1981.tb00667.x
- Adamopoulos DA, Nicopoulou S, Kapolla N, Vassilopoulos P, Karamertzanis M, Kontogeorgos L. Endocrine effects of testosterone undecanoate as a supplementary treatment to menopausal gonadotropins or tamoxifen citrate in idiopathic oligozoospermia. Fertility and Sterility. 1995;64(4):818-824. doi:10.1016/s0015-0282(16)57860-1
- Gårevik N, Rane A, Björkhem‐Bergman L, Ekström L. Effects of different doses of testosterone on gonadotropins, 25-hydroxyvitamin D3, and blood lipids in healthy men. Substance Abuse and Rehabilitation. Published online December 1, 2014:121. doi:10.2147/sar.s71285
- Desai A, Yassin M, Cayetano A, Tharakan T, Jayasena C. Understanding and managing the suppression of spermatogenesis caused by testosterone replacement therapy (TRT) and anabolic–androgenic steroids (AAS). Therapeutic Advances in Urology. 2022;14:175628722211050. doi:10.1177/17562872221105017
- Swerdloff RS, Wang C, White WΒ, et al. A New Oral Testosterone Undecanoate Formulation Restores Testosterone to Normal Concentrations in Hypogonadal Men. The Journal of Clinical Endocrinology & Metabolism. 2020;105(8):2515-2531. doi:10.1210/clinem/dgaa238
- Ahn H, Park JH. Liposomal delivery systems for intestinal lymphatic drug transport. Biomaterials Research. 2016;20(1). doi:10.1186/s40824-016-0083-1
- Eckman A, Dobs AS. Drug-induced gynecomastia. Expert Opinion on Drug Safety. 2008;7(6):691-702. doi:10.1517/14740330802442382
- Plymate SR, Leonard JM, Paulsen CA, Fariss BL, Karpas AE. Sex Hormone-Binding Globulin Changes with Androgen Replacement. The Journal of Clinical Endocrinology & Metabolism. 1983;57(3):645-648. doi:10.1210/jcem-57-3-645
- Oduwole O, Huhtaniemi I, Misrahi M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. International Journal of Molecular Sciences. 2021;22(23):12735. doi:10.3390/ijms222312735
- Yin A, Alfadhli E, Htun M, et al. Dietary Fat Modulates the Testosterone Pharmacokinetics of a New Self‐Emulsifying Formulation of Oral Testosterone Undecanoate in Hypogonadal Men. Andrology. 2012;33(6):1282-1290. doi:10.2164/jandrol.112.017020
- Rodríguez K, Pastuszak AW, Lipshultz LI. Enclomiphene citrate for the treatment of secondary male hypogonadism. Expert Opinion on Pharmacotherapy. 2016;17(11):1561-1567. doi:10.1080/14656566.2016.1204294
- Newell-Price J, Huatan H, Quirke J, et al. An oral lipidic native testosterone formulation that is absorbed independent of food. Eur J Endocrinol. 2021;185(5):607-615. Published 2021 Oct 5. doi:10.1530/EJE-21-0606
- Pastuszak AW, Gittelman M, Tursi JP, Jaffe JS, Schofield D, Miner M. Pharmacokinetics of testosterone therapies in relation to diurnal variation of serum testosterone levels as men age. Andrology. 2021;10(2):209-222. doi:10.1111/andr.13108
- Platz EA, Barber JR, Chadid S, et al. Nationally Representative Estimates of Serum Testosterone Concentration in Never-Smoking, Lean Men Without Aging-Associated Comorbidities. Journal of the Endocrine Society. 2019;3(10):1759-1770. doi:10.1210/js.2019-00151
- Luteinizing hormone (LH) blood test. Mount Sinai Health System. https://www.mountsinai.org/health-library/tests/luteinizing-hormone-lh-blood-test
- Follicle-stimulating hormone (FSH) blood test. Mount Sinai Health System. https://www.mountsinai.org/health-library/tests/follicle-stimulating-hormone-fsh-blood-test
- Sikaris K, McLachlan RI, Kazlauskas R, De Kretser DM, Holden CA, Handelsman DJ. Reproductive hormone reference intervals for healthy fertile young men: Evaluation of Automated platform assays. The Journal of Clinical Endocrinology & Metabolism. 2005;90(11):5928-5936. doi:10.1210/jc.2005-0962
- Löwe B, Wahl I, Rose M, et al. A 4-item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. Journal of Affective Disorders. 2010;122(1-2):86-95. doi:10.1016/j.jad.2009.06.019
- Mohamed OG, Freundlich RE, Dakik HK, et al. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. International Journal of Impotence Research. 2009;22(1):20-24. doi:10.1038/ijir.2009.35
- Straftis A, Gray PB. Sex, Energy, Well-Being and Low Testosterone: An Exploratory Survey of U.S. Men’s Experiences on Prescription Testosterone. International Journal of Environmental Research and Public Health. 2019;16(18):3261. doi:10.3390/ijerph16183261
- Zitzmann M. Testosterone, mood, behaviour and quality of life. Andrology. 2020;8(6):1598-1605. doi:10.1111/andr.12867
- Kutlikova HH, Geniole SN, Eisenegger C, Lamm C, Jocham G, Studer B. Not giving up: Testosterone promotes persistence against a stronger opponent. Psychoneuroendocrinology. 2021;128:105214. doi:10.1016/j.psyneuen.2021.105214
- McIntyre RS, Mancini D, Eisfeld BS, et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31(9):1029-1035. doi:10.1016/j.psyneuen.2006.06.005
- Walther A, Breidenstein J, Miller R. Association of Testosterone Treatment With Alleviation of Depressive Symptoms in Men. JAMA Psychiatry. 2019;76(1):31. doi:10.1001/jamapsychiatry.2018.2734
Supplementary Material

PHQ-4 questionnaire (Supplementary Material 1)
The PHQ-4 is a brief screening questionnaire that assesses anxiety and depression.23 Participants are asked to rate the frequency of the following symptoms over the last two weeks, to the point of being bothered:
- Feeling nervous, anxious, or on edge
- Not being able to stop or control worrying
- Feeling down, depressed or hopeless
- Little interest or pleasure in doing things
Responses are captured on a simple four response scale with the following point allocation:
- Not at all = 0
- Several days = 1
- More than half the days = 2
- Nearly every day = 3
Response points are summed to determine overall function: normal (0-2), mild (3-5), moderate (6-8), and severe (9-12).
The responses to the first two questions are summed to assess anxiety, where a score >=3 suggests anxiety.
The responses to the second two questions are summed to assess depression, where a score >=3 suggests depression.

qADAM questionnaire (Supplementary Material 2)
The qADAM responses generate a composite score ranging from 10-50, with a lower score being indicative of potential hypogonadism.22 Responses to each of the questions are collected on a five-point scale.
The qADAM questionnaire is as follows:
- How would you rate your energy level?
- How would you rate your strength/endurance?
- How would you rate your enjoyment of life?
- How would you rate your happiness level?
- How would you rate your libido (sex drive)?
- How strong are your erections?
- How would you rate your work performance over the last 4 weeks?
- How often do you fall asleep after dinner without intending to?
- How would you rate your sports ability over the last 4 weeks?
- How much height have you lost in your lifetime?
For all questions, with the exception of the question related to height loss, response scoring is as follows:
- Terrible = 1
- Poor = 2
- Average = 3
- Good = 4
- Excellent = 5
Responses of 3 (Average), or higher were categorized as normal function. Total qADAM composite scores of 30 or higher (indicating an average response of 3 or higher per question) were categorized as normal function.
Baseline height loss responses are assumed constant for follow-up, given participants could not reasonably lose additional height within the time duration of the study.
Distribution of Treatments

Post Treatment LH and FSH
Histogram of Post Treatment across all experiments (n=79)
Post Treatment across all experiments
LH
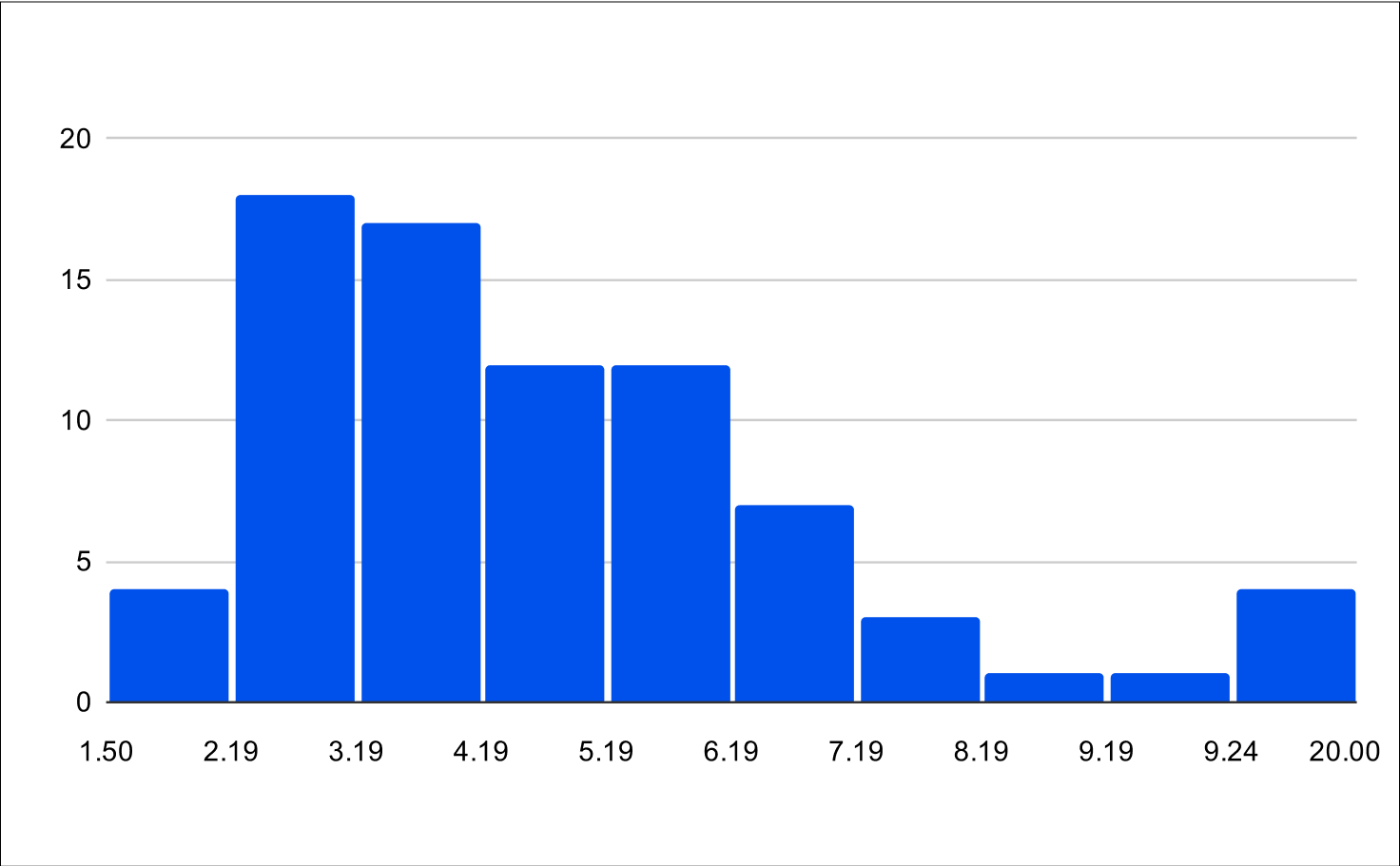
FSH

Pilot B Distribution
Baseline Total Testosterone
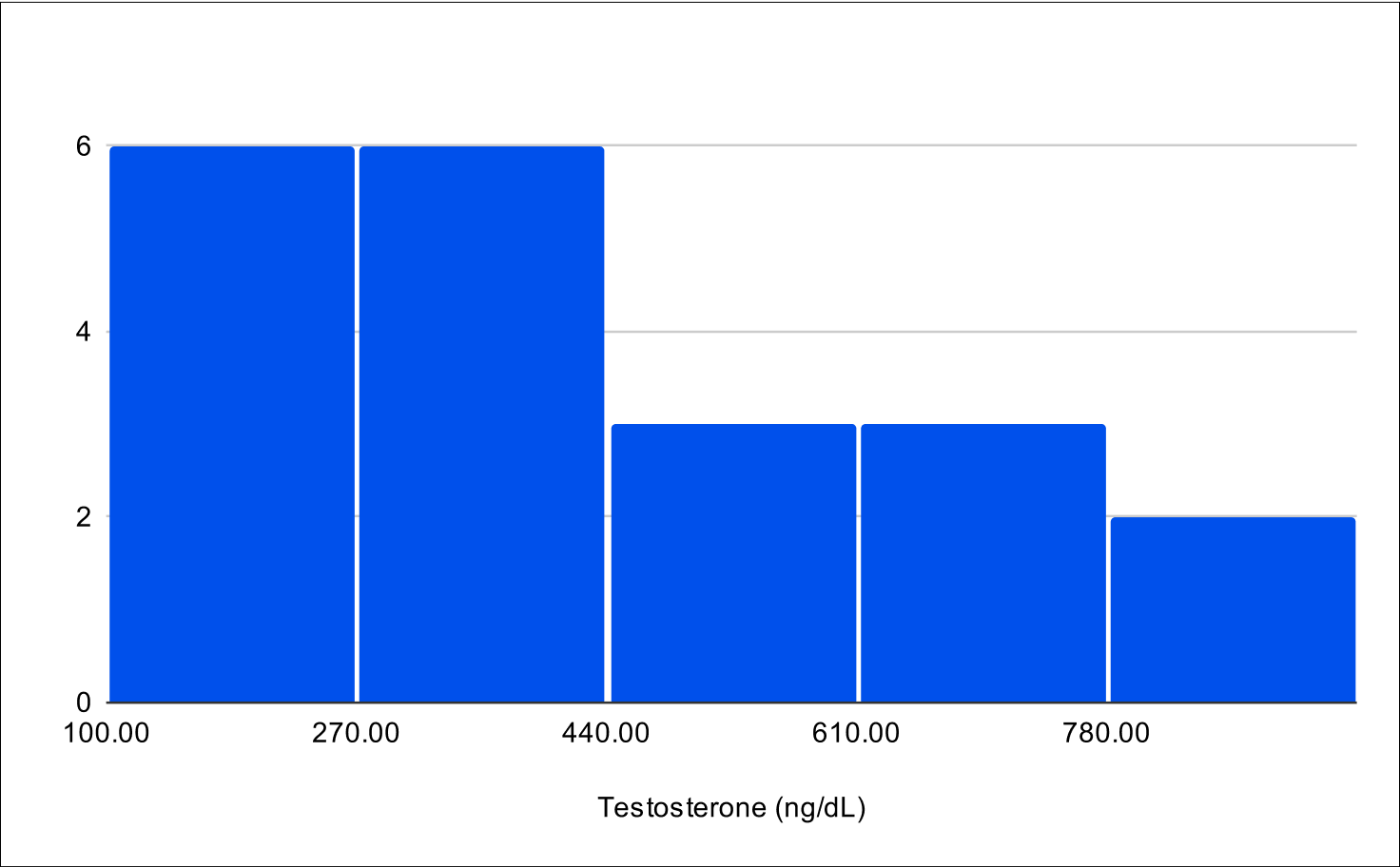
Baseline Free Testosterone
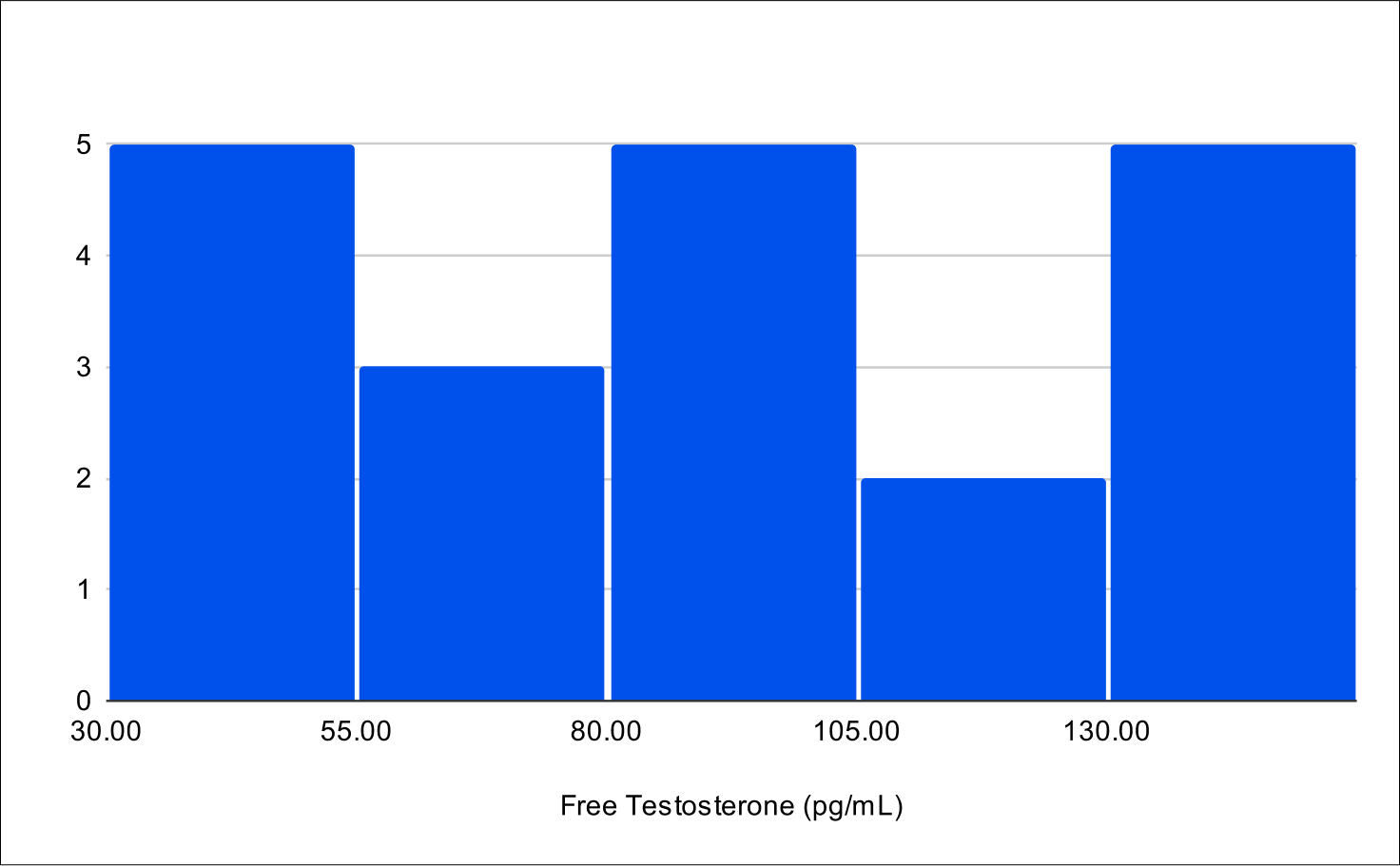
Baseline FSH
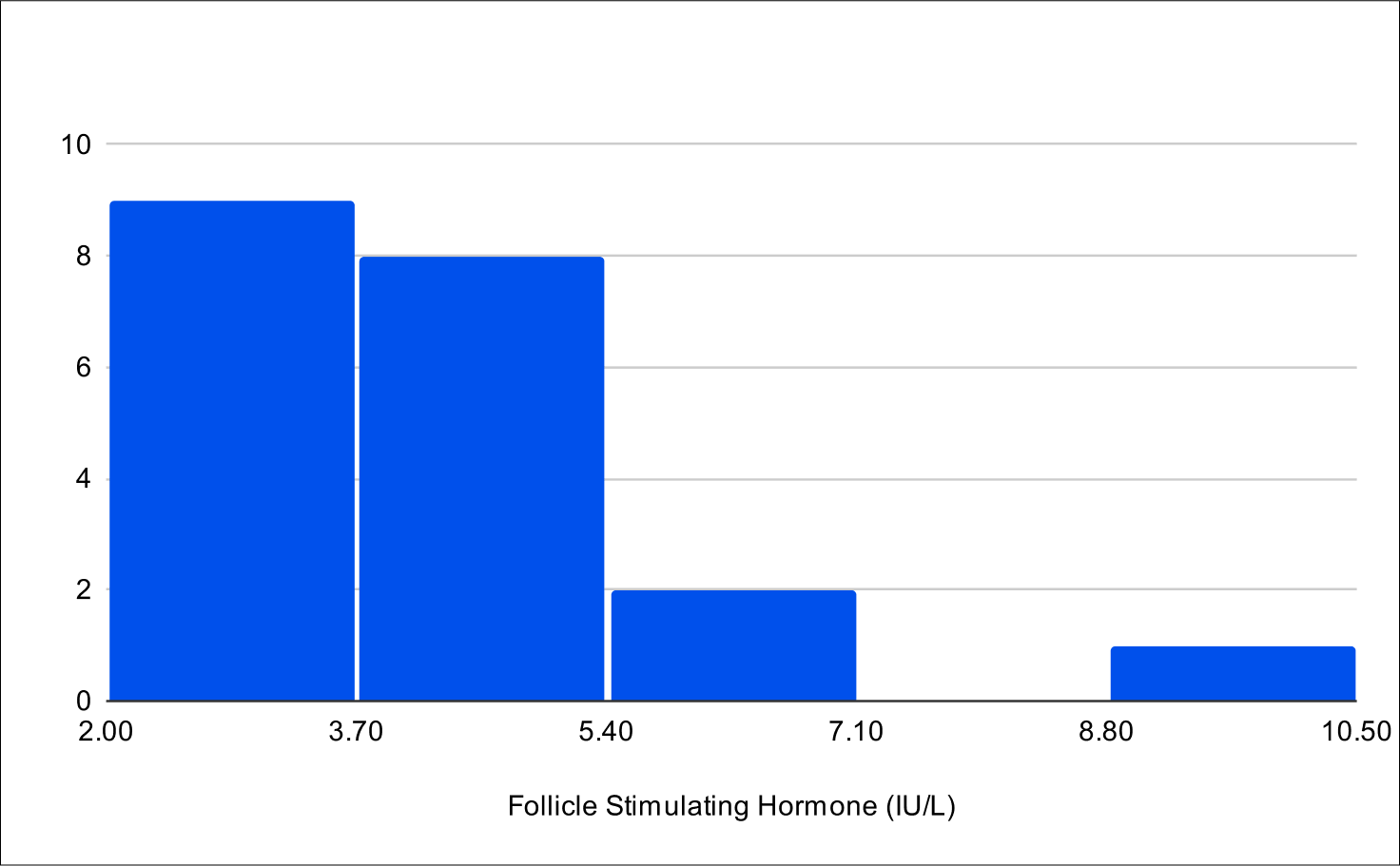
Baseline LH
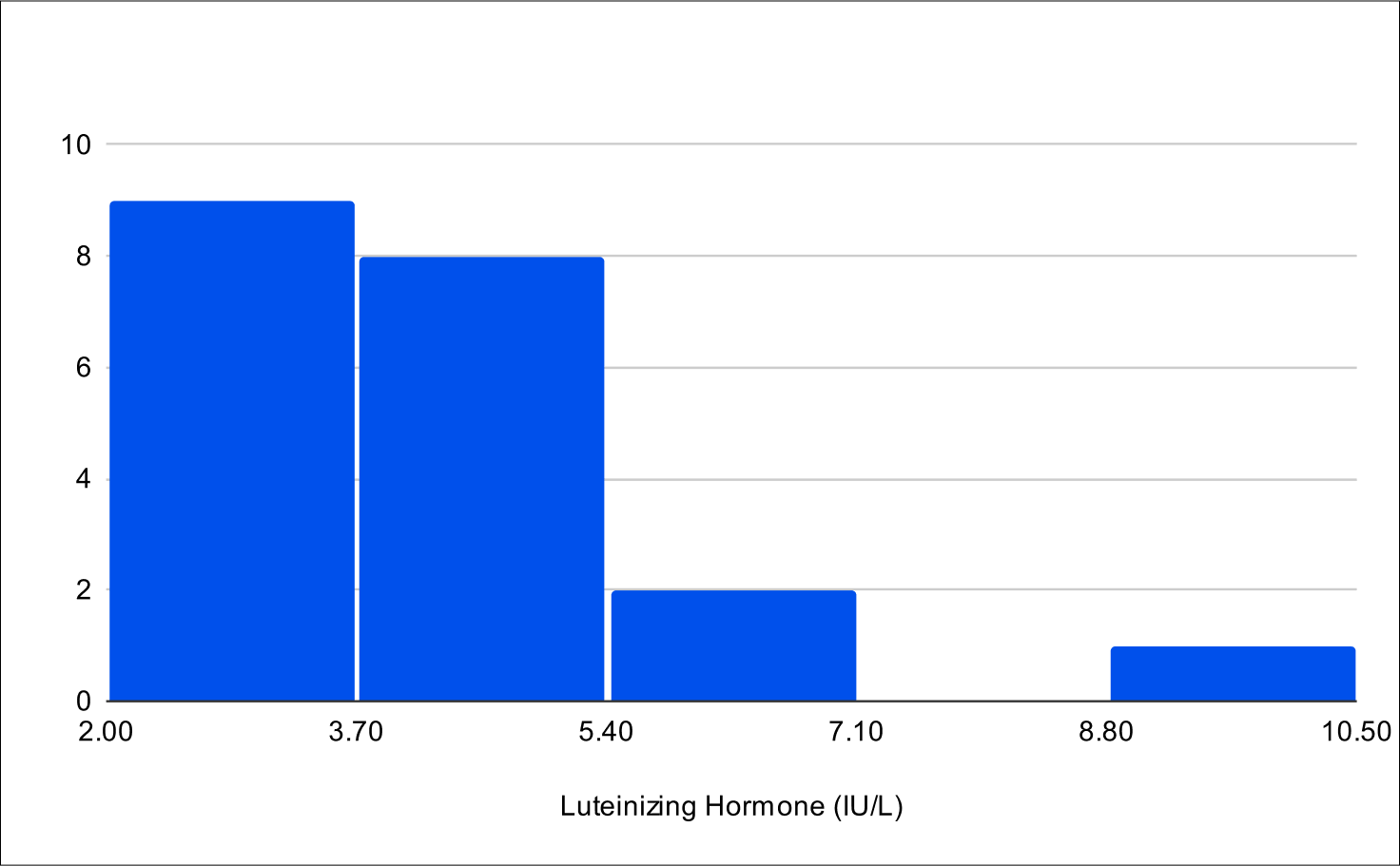
Baseline E2
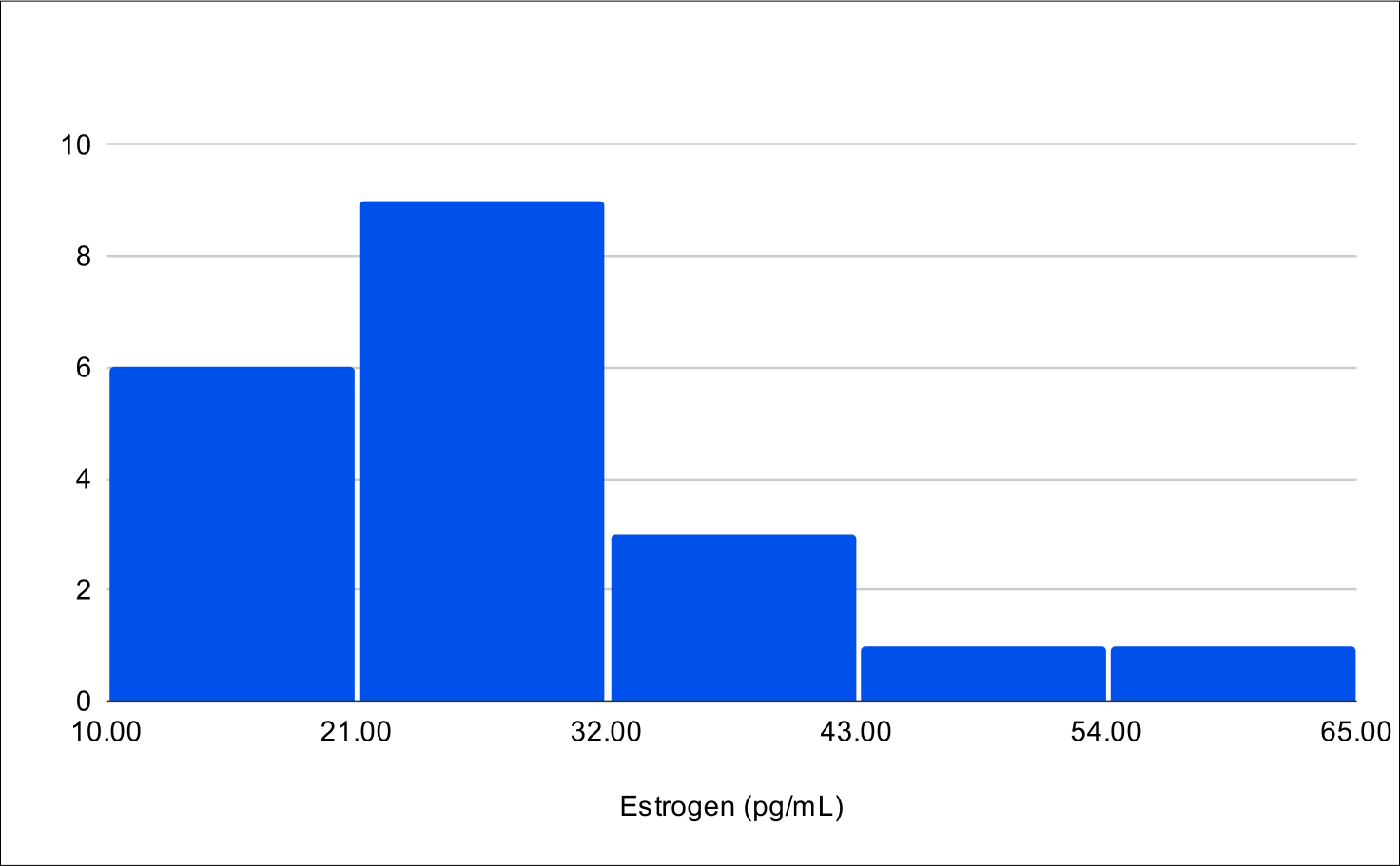
Baseline SHBG
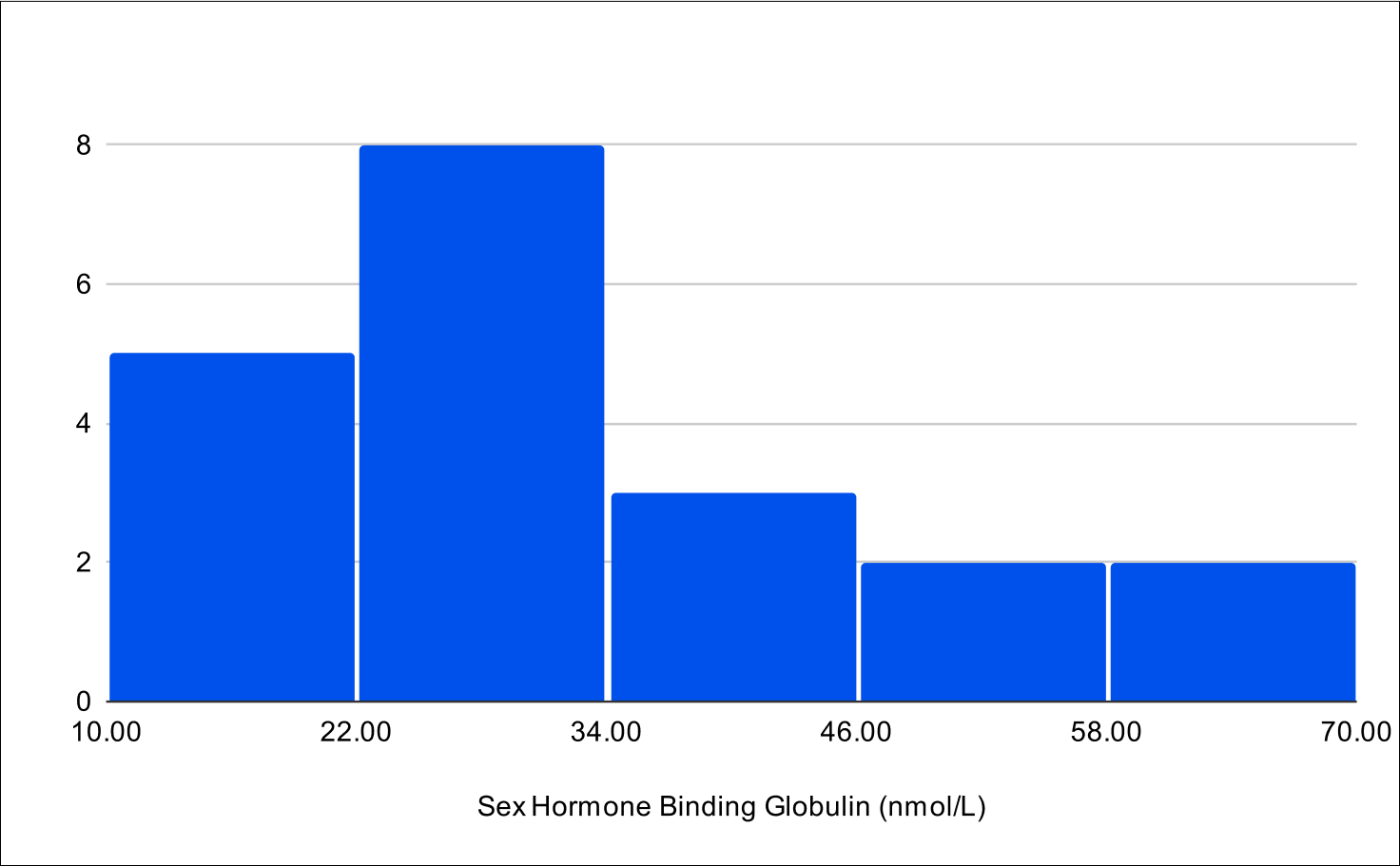
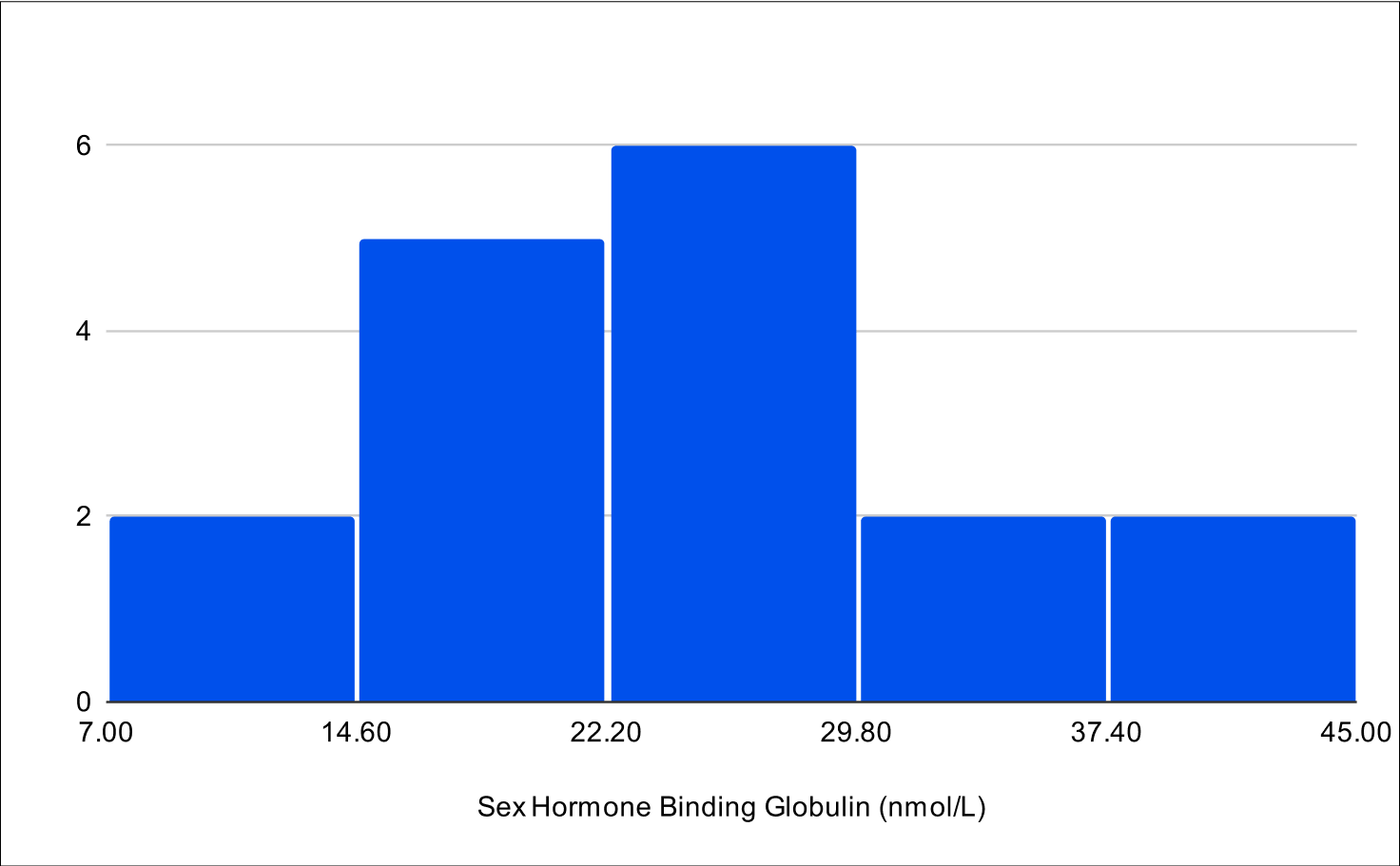
BF+4 Distribution
Baseline Total Testosterone
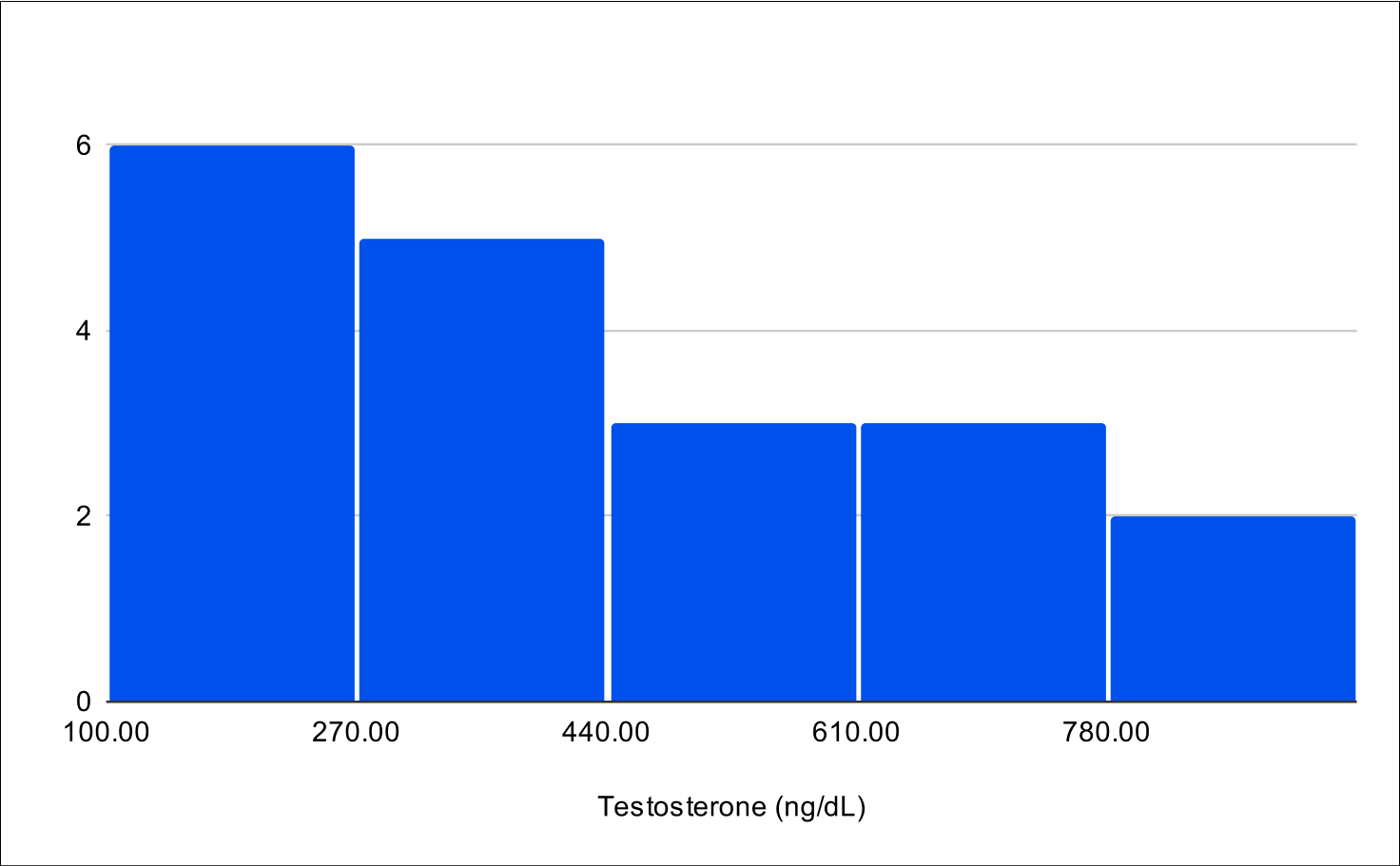
Baseline Free Testosterone
Baseline FSH
Baseline LH
Baseline E2
Baseline SHBG
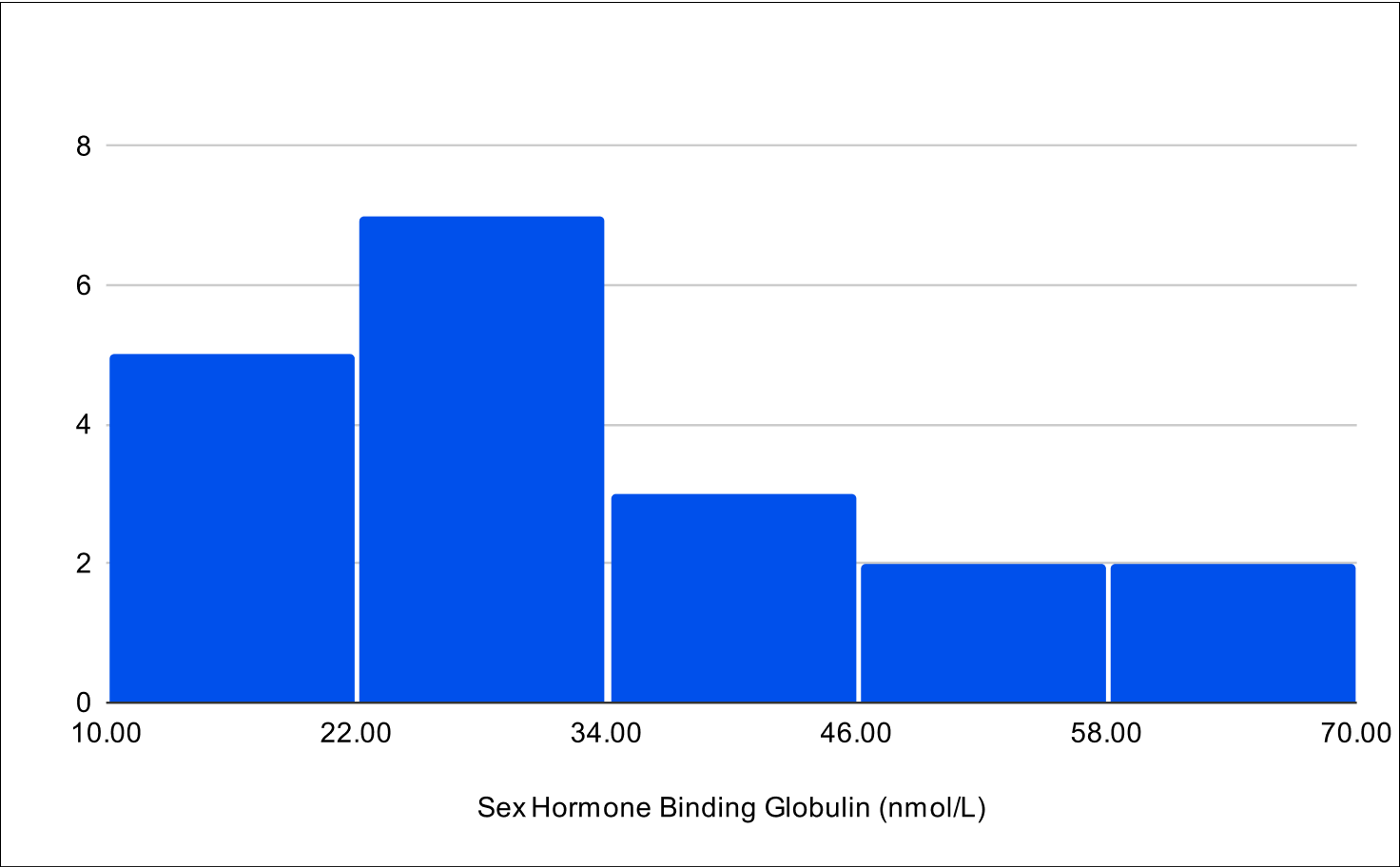
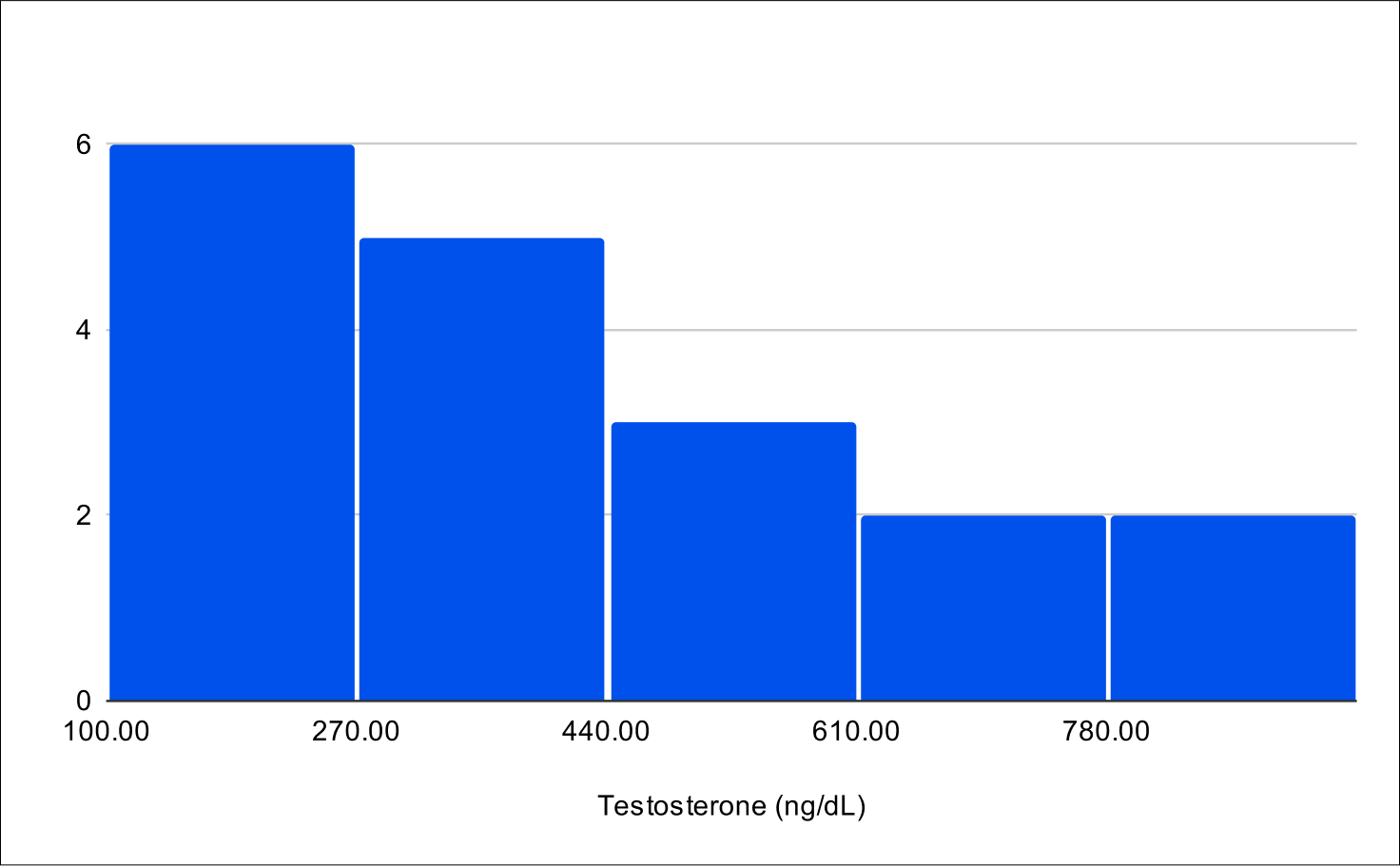
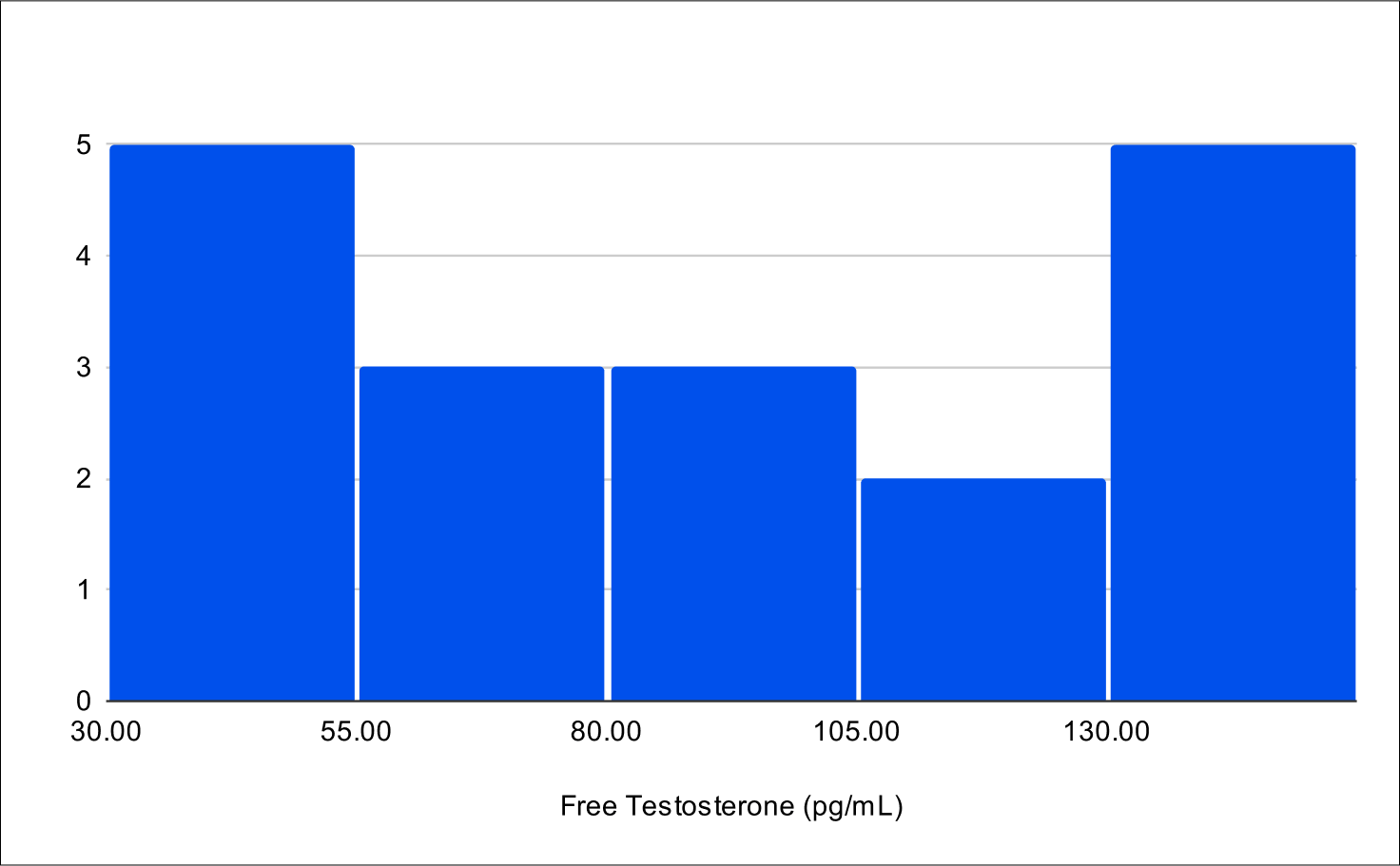
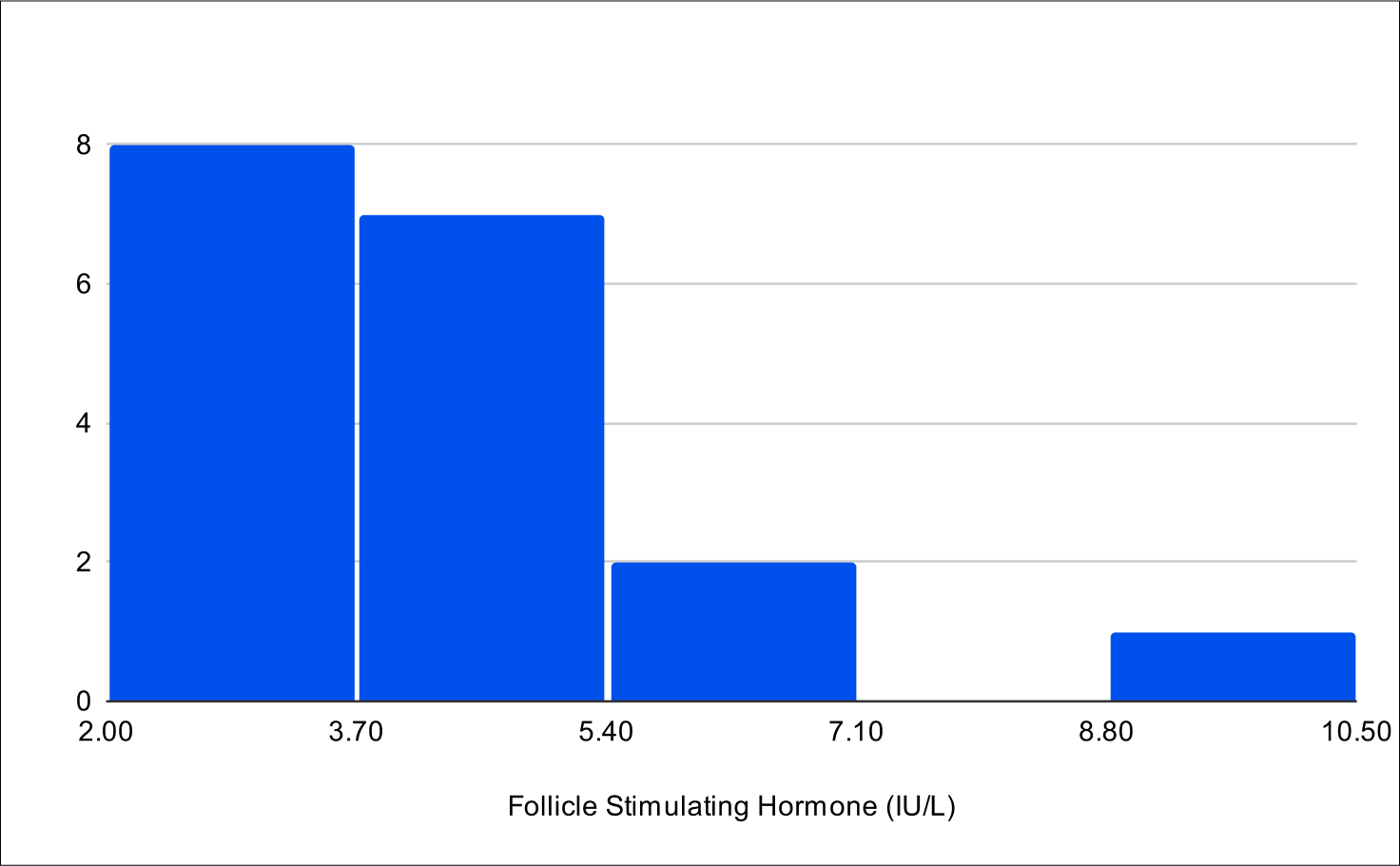
B+1 Distribution
Baseline Total Testosterone
Baseline Free Testosterone
Baseline FSH
Baseline LH
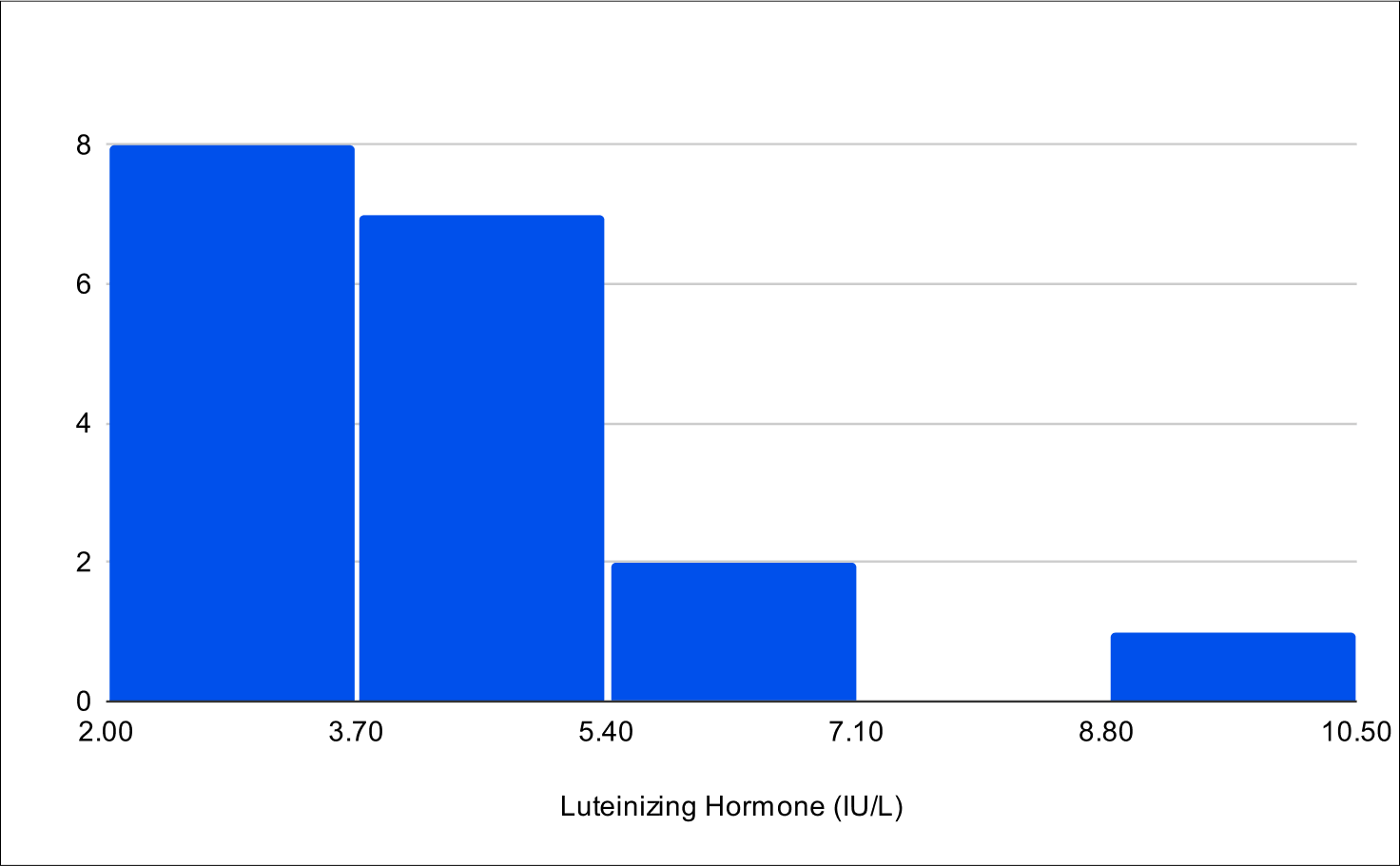
Baseline E2
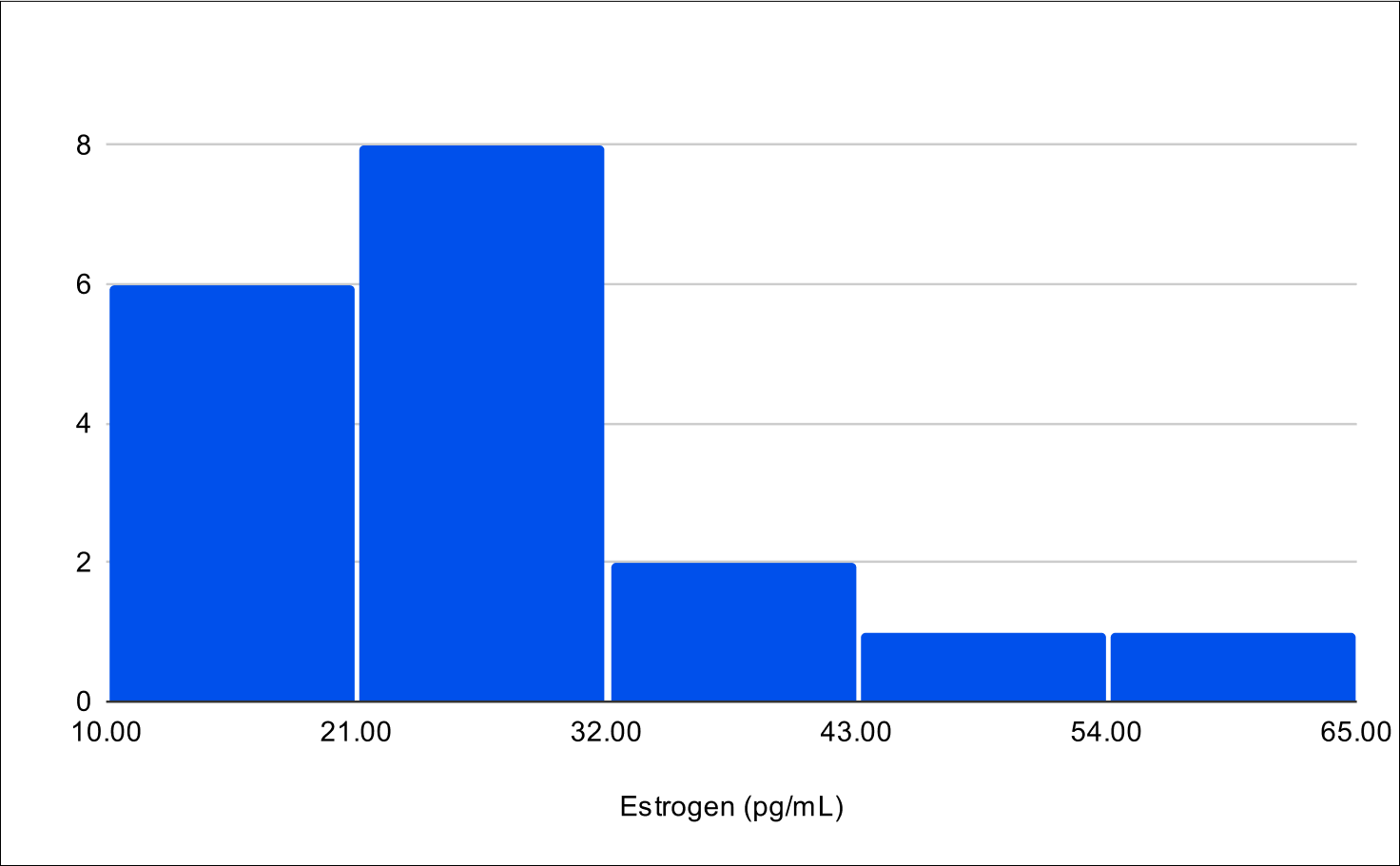
Baseline SHBG
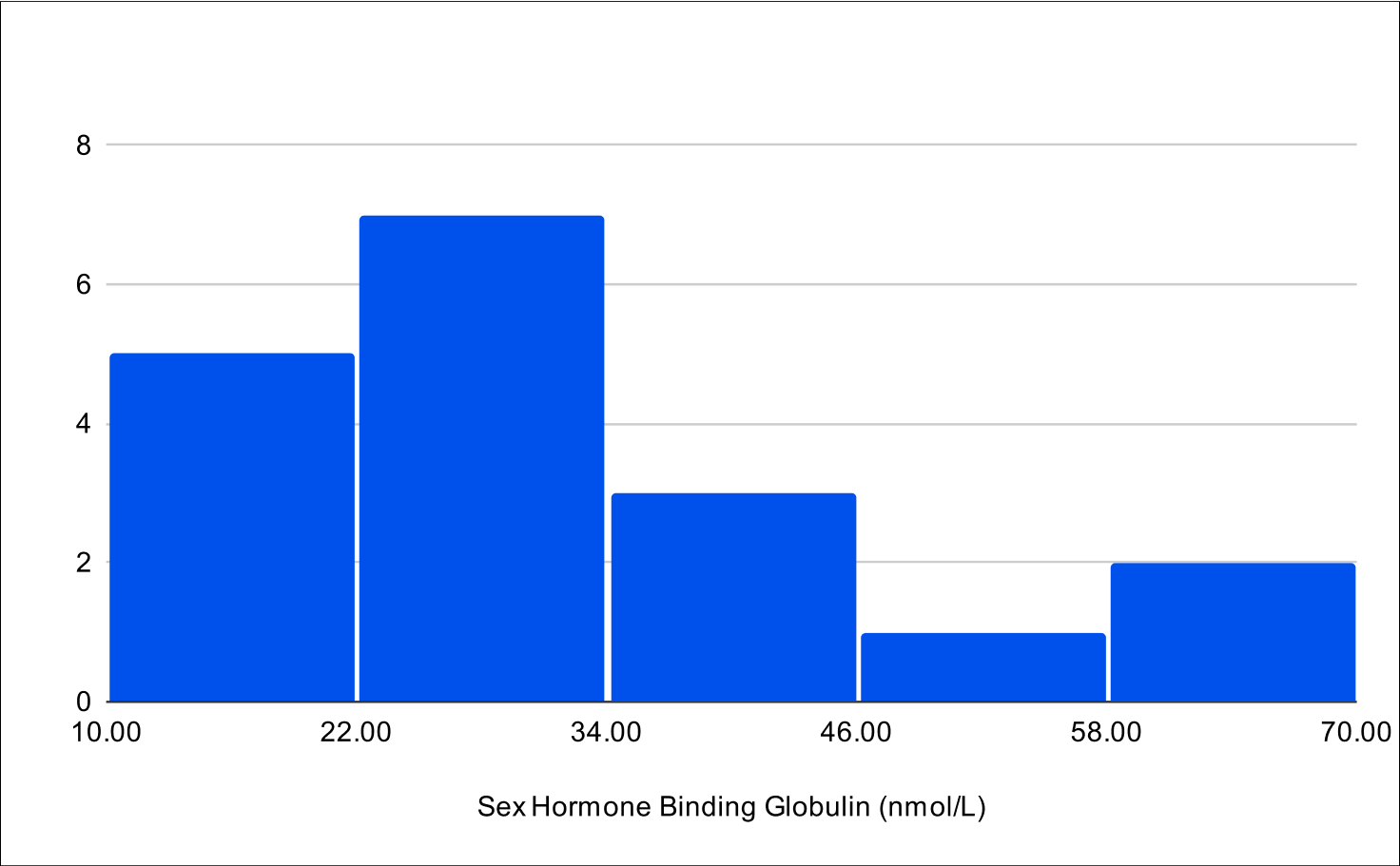
B+4 Distribution
Baseline Total Testosterone
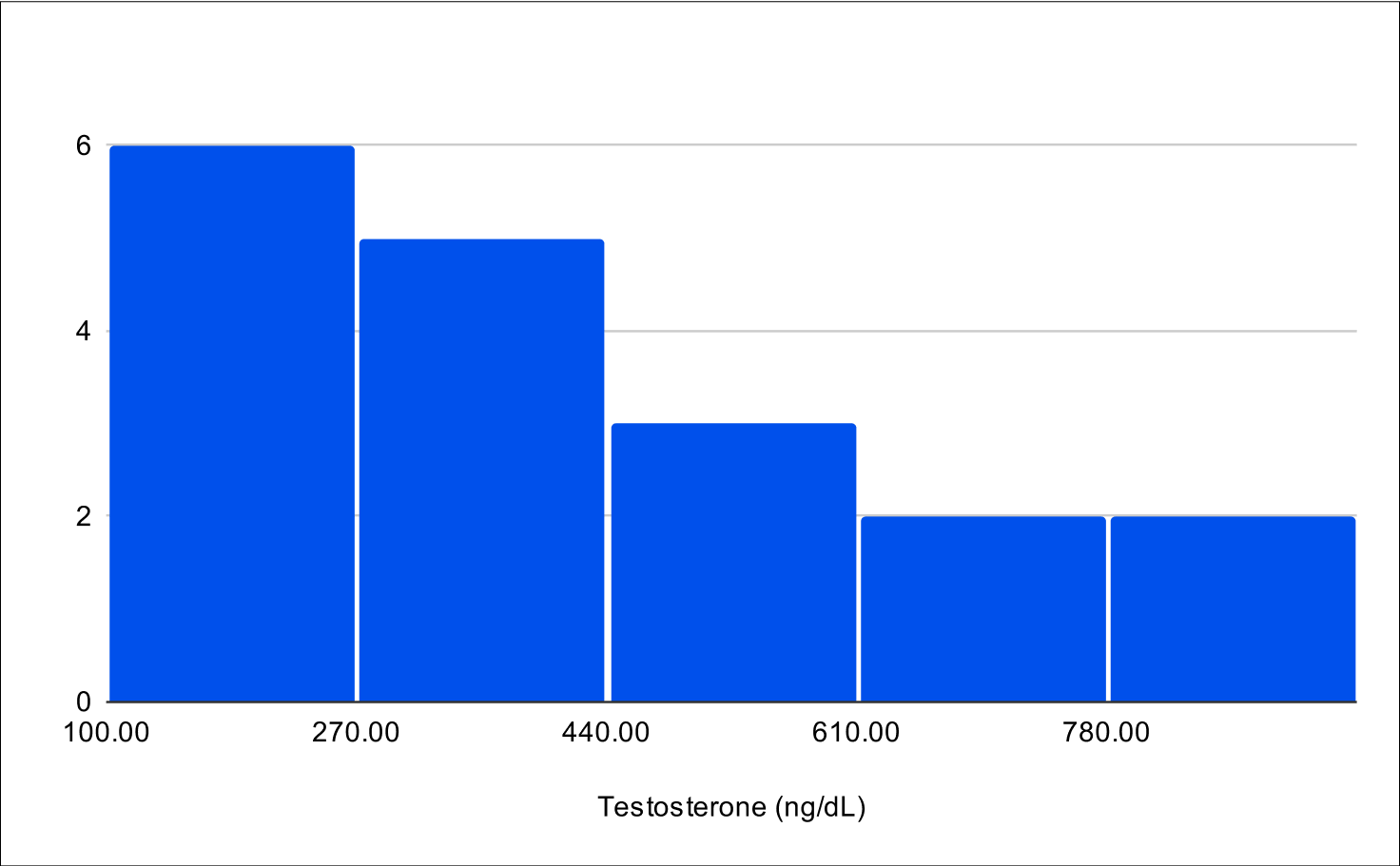
Baseline Free Testosterone
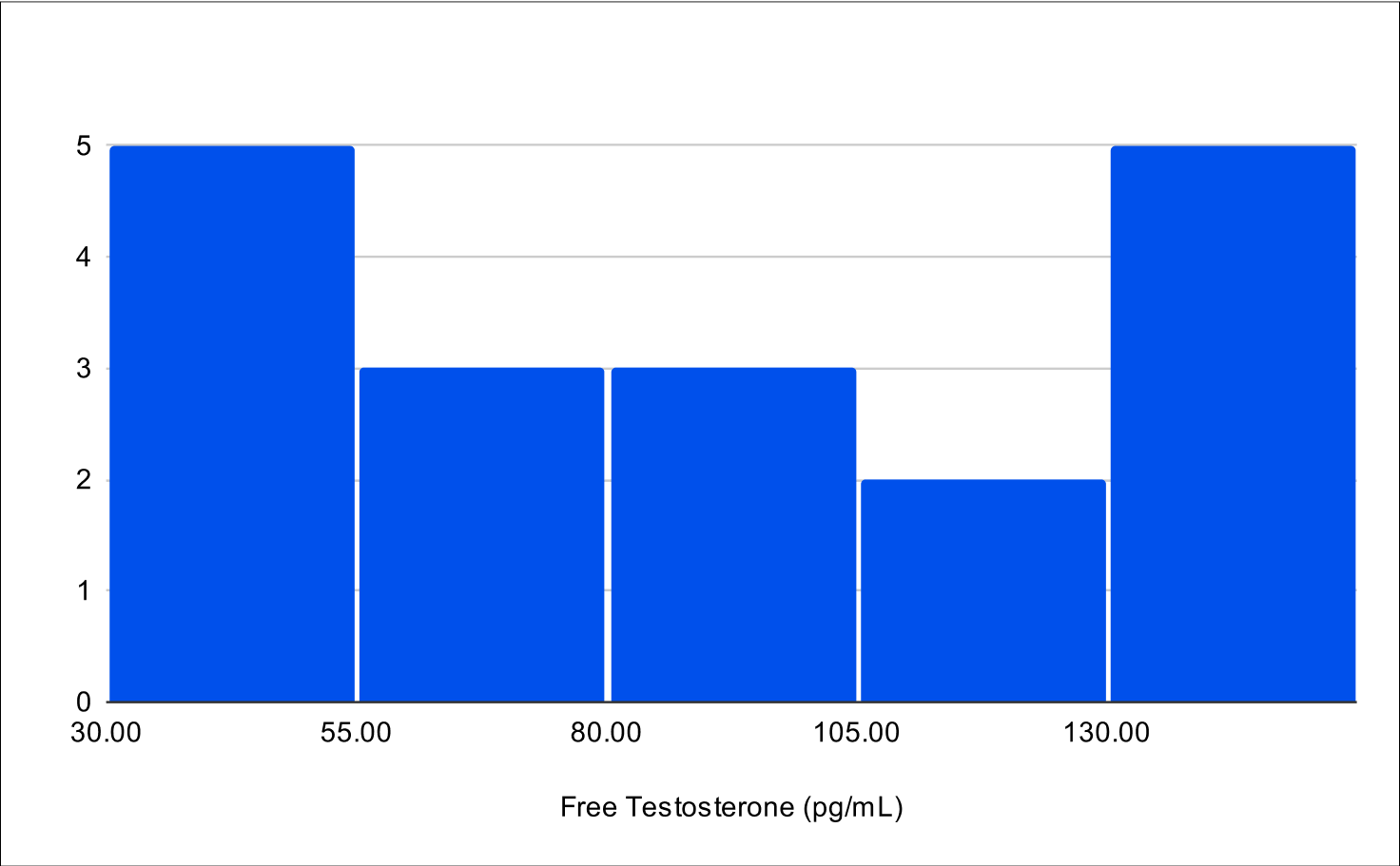
Baseline FSH
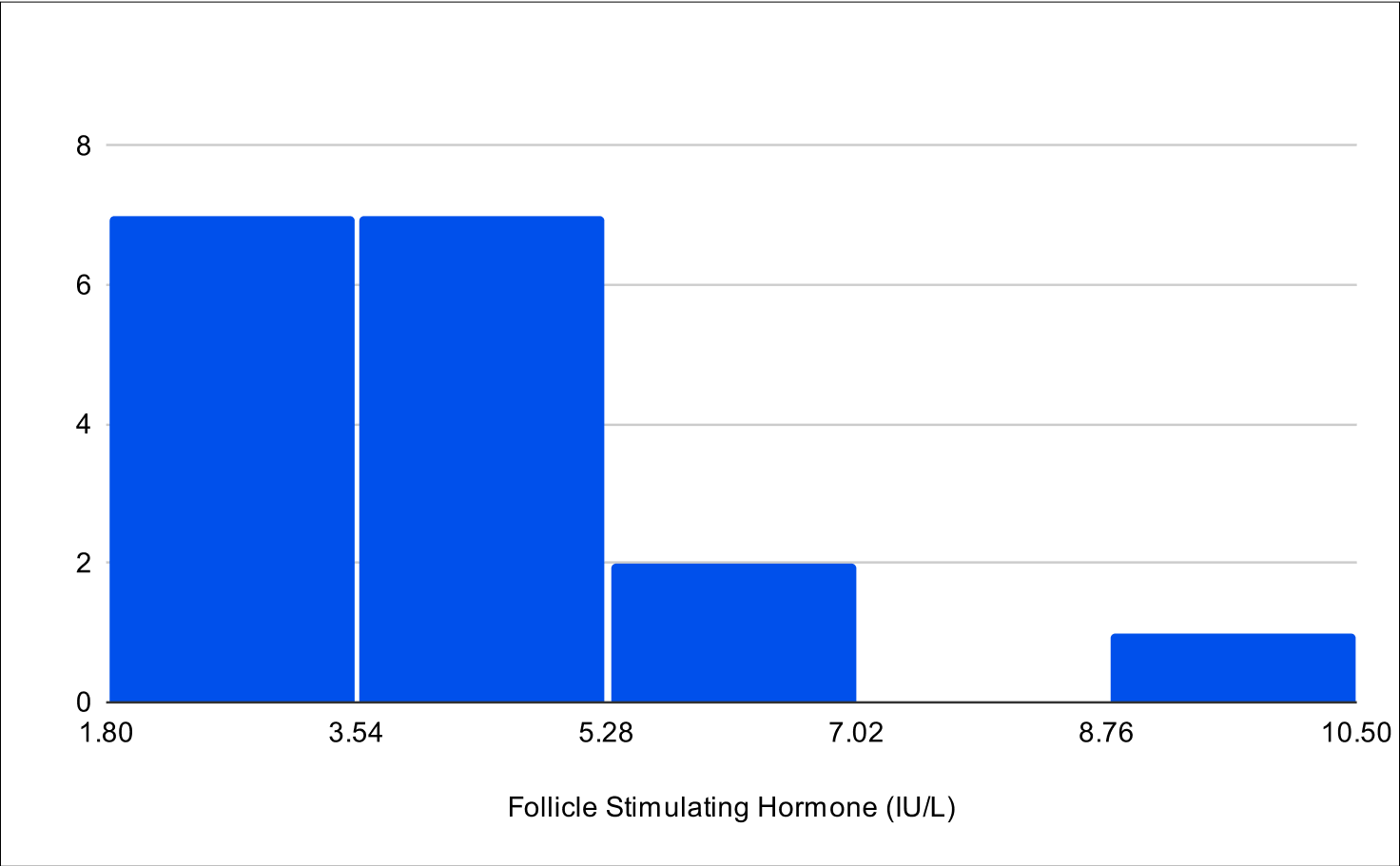
Baseline LH
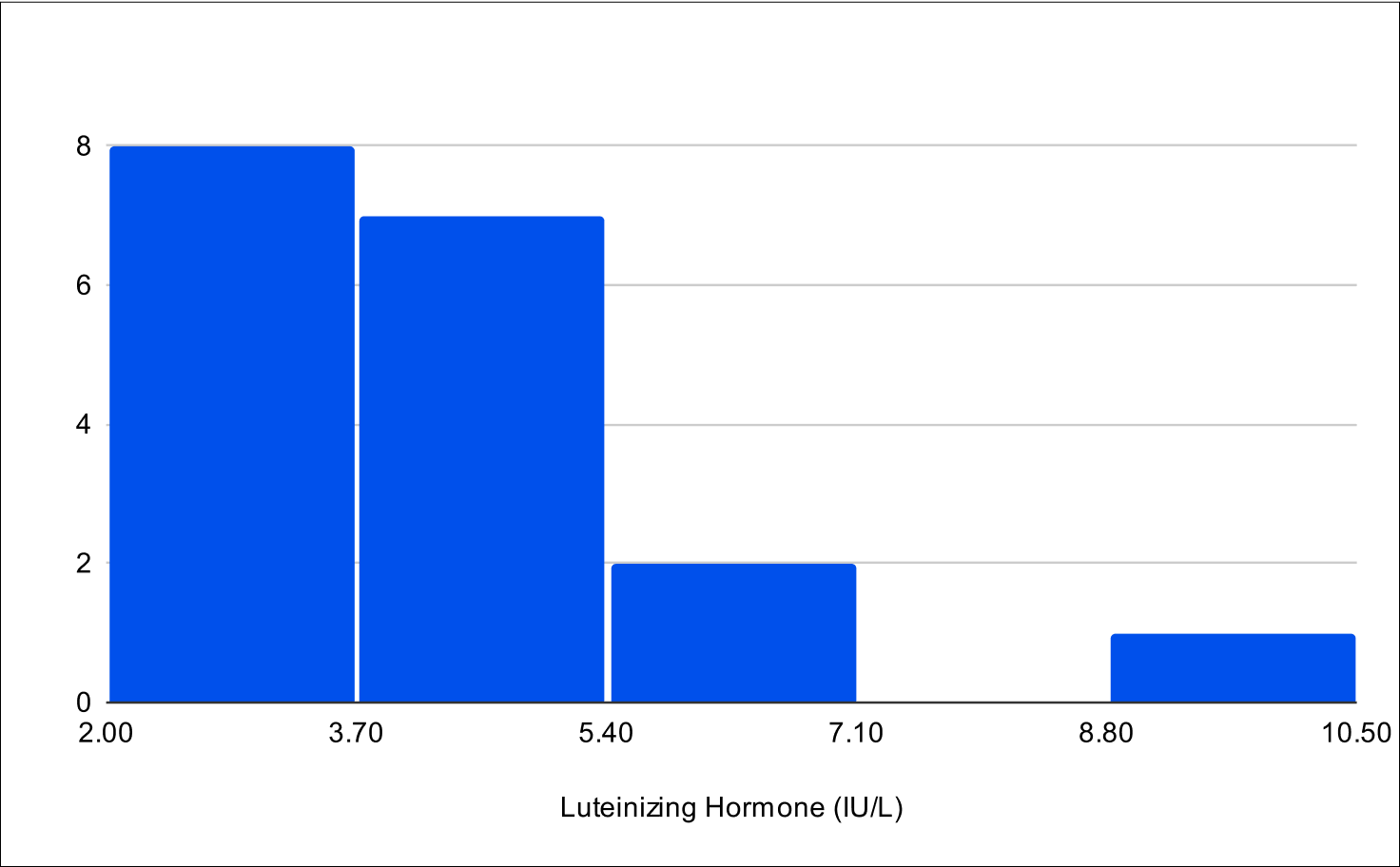
Baseline E2
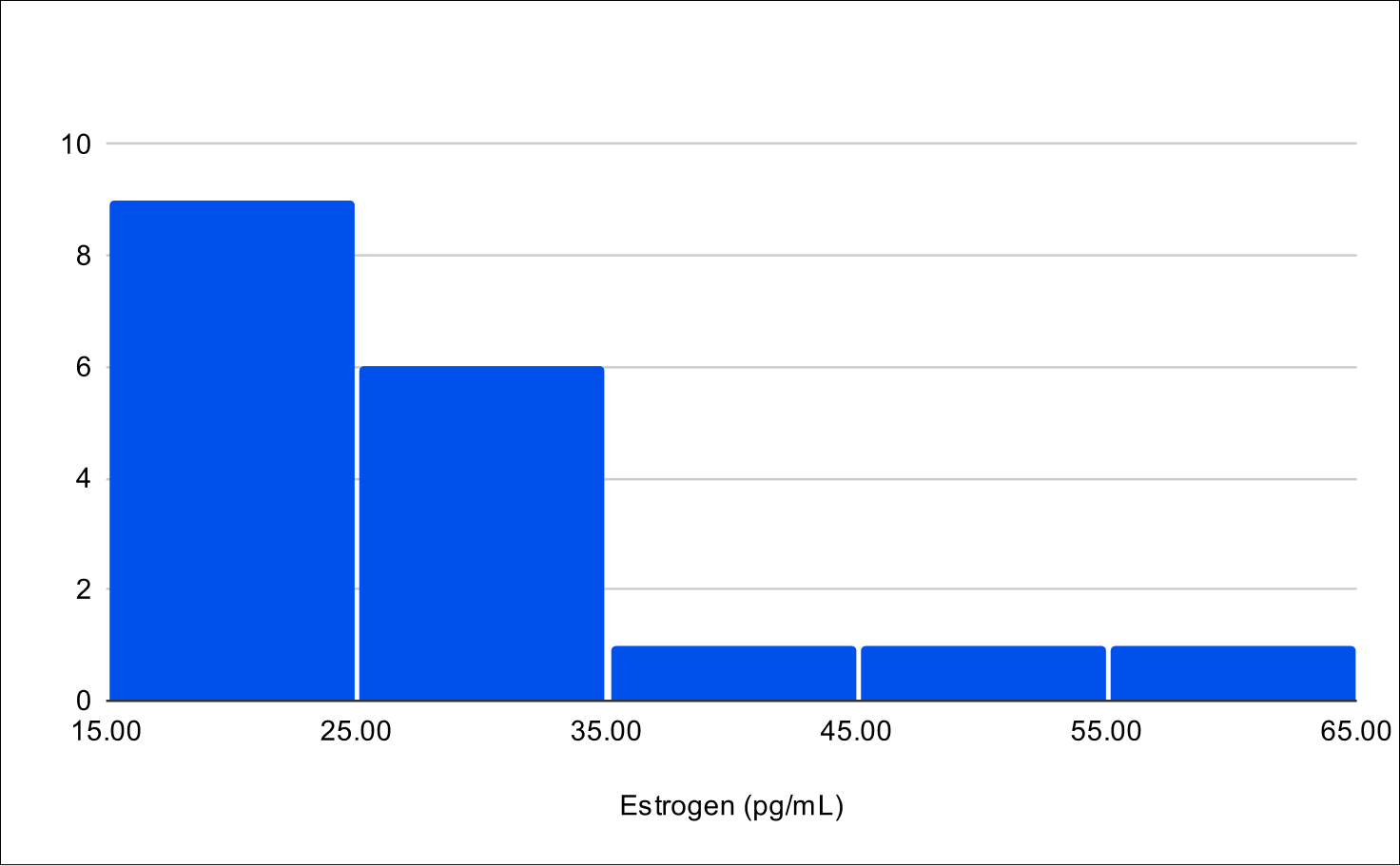
Baseline SHBG
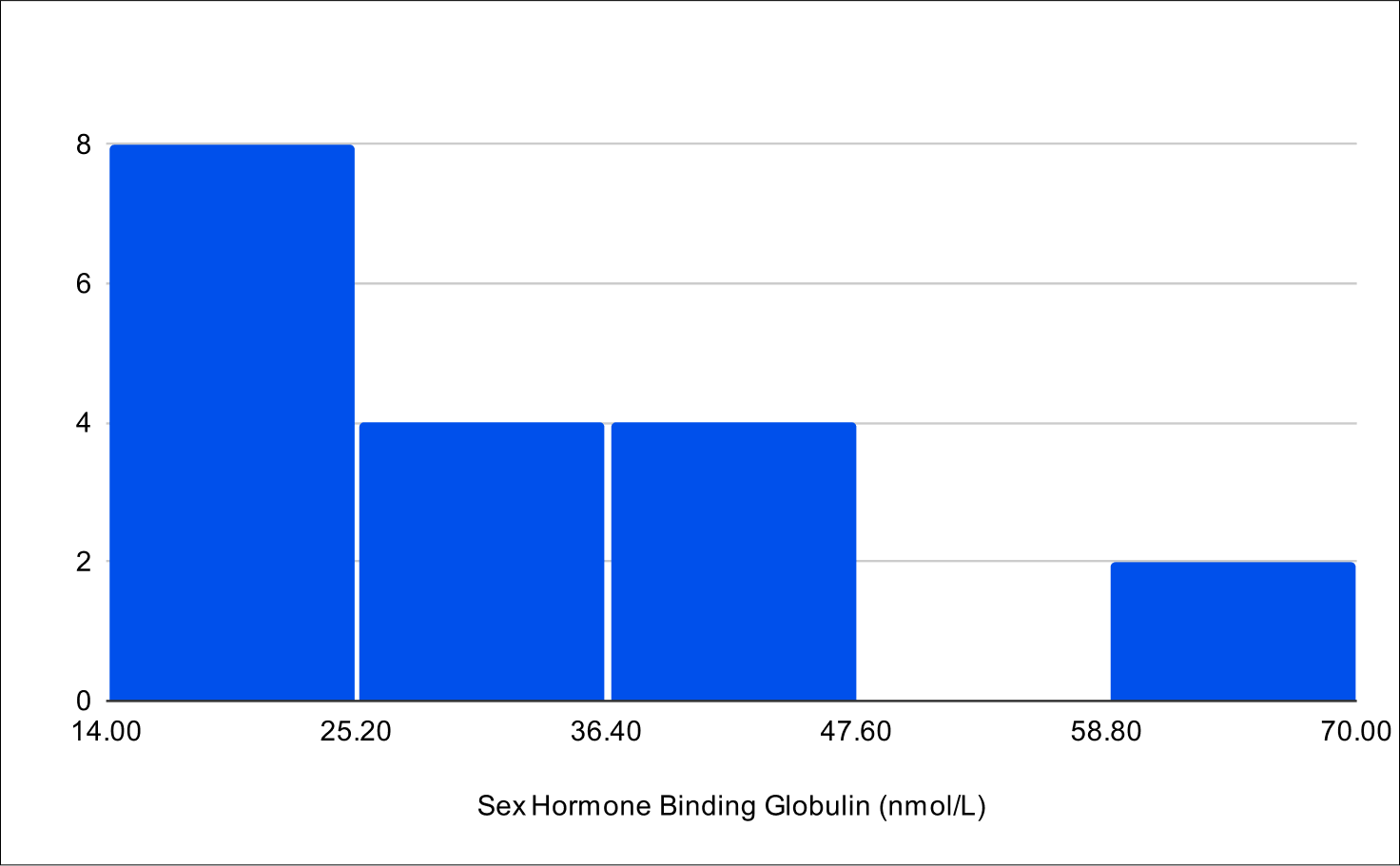
B+4 Post Treatment Distribution
Baseline Total Testosterone
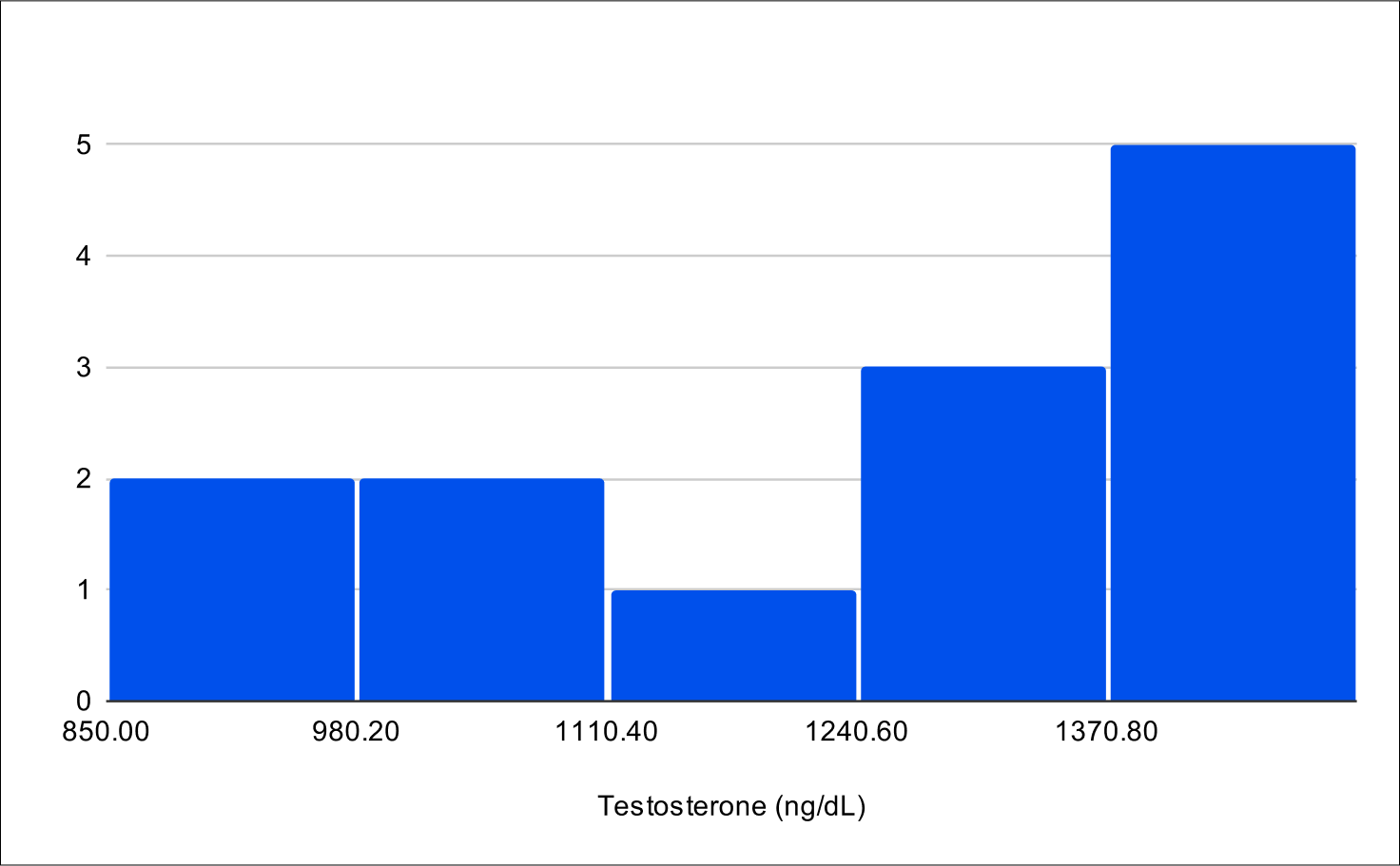
Baseline Free Testosterone
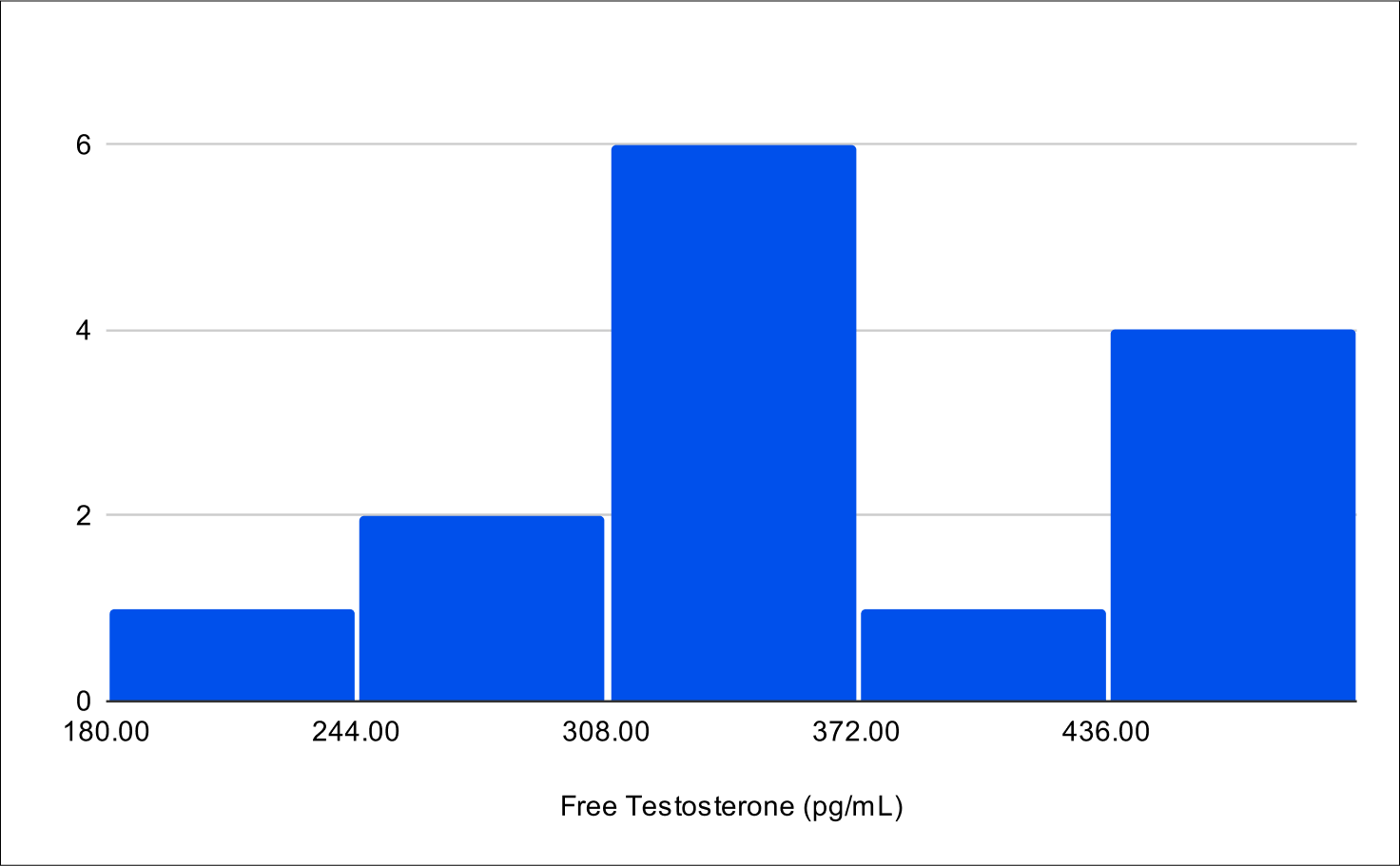
Baseline FSH
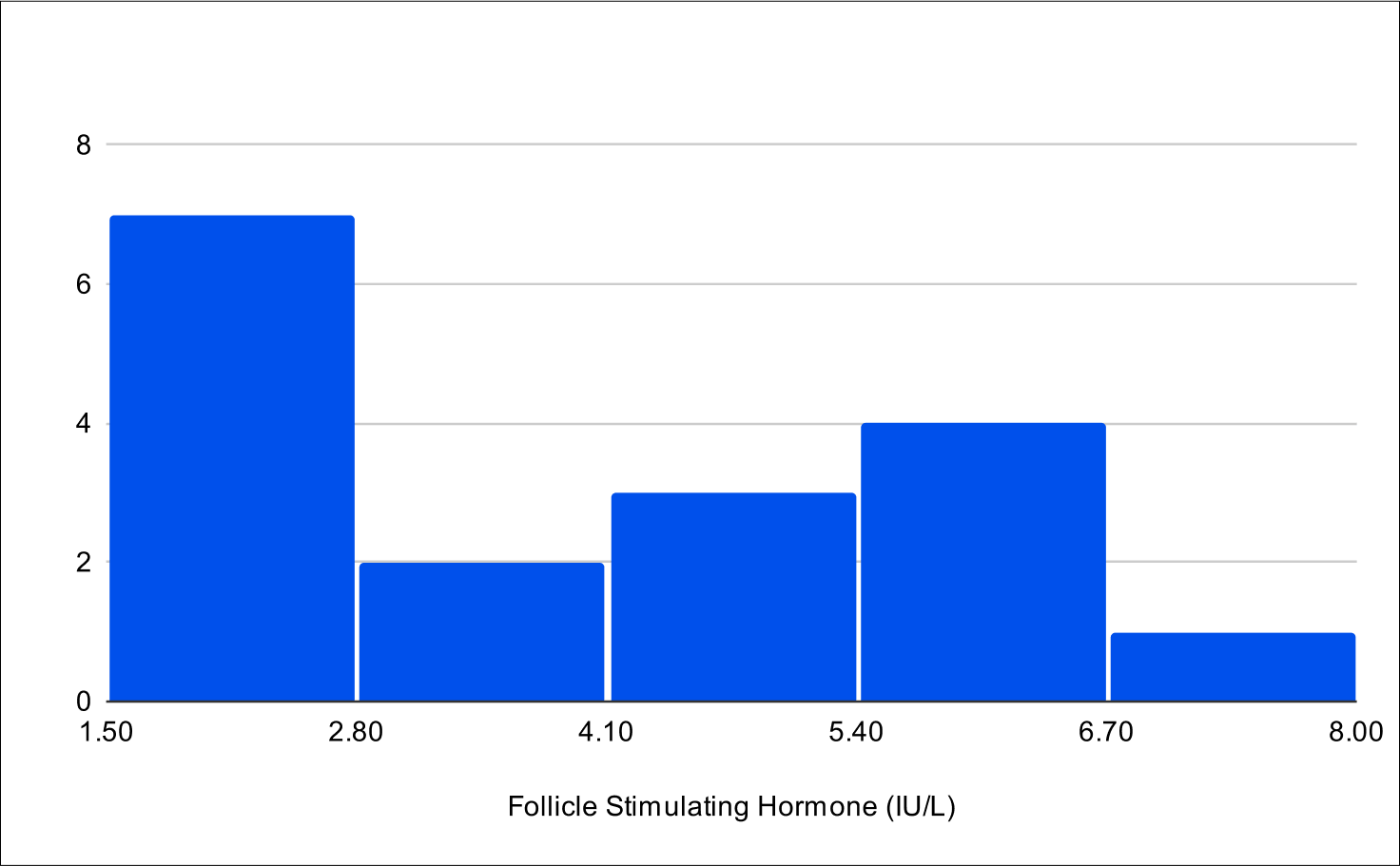
Baseline LH
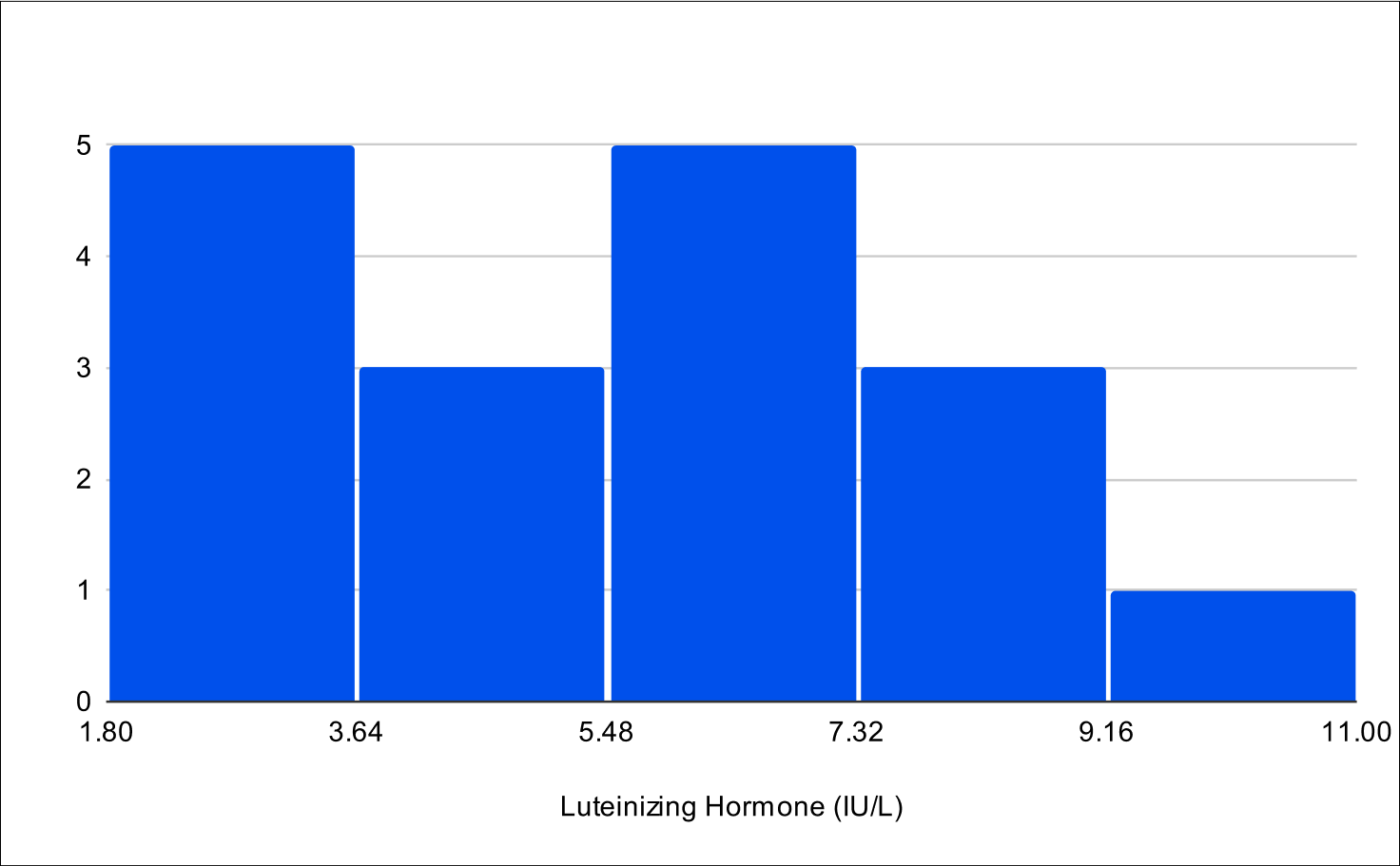
Baseline E2
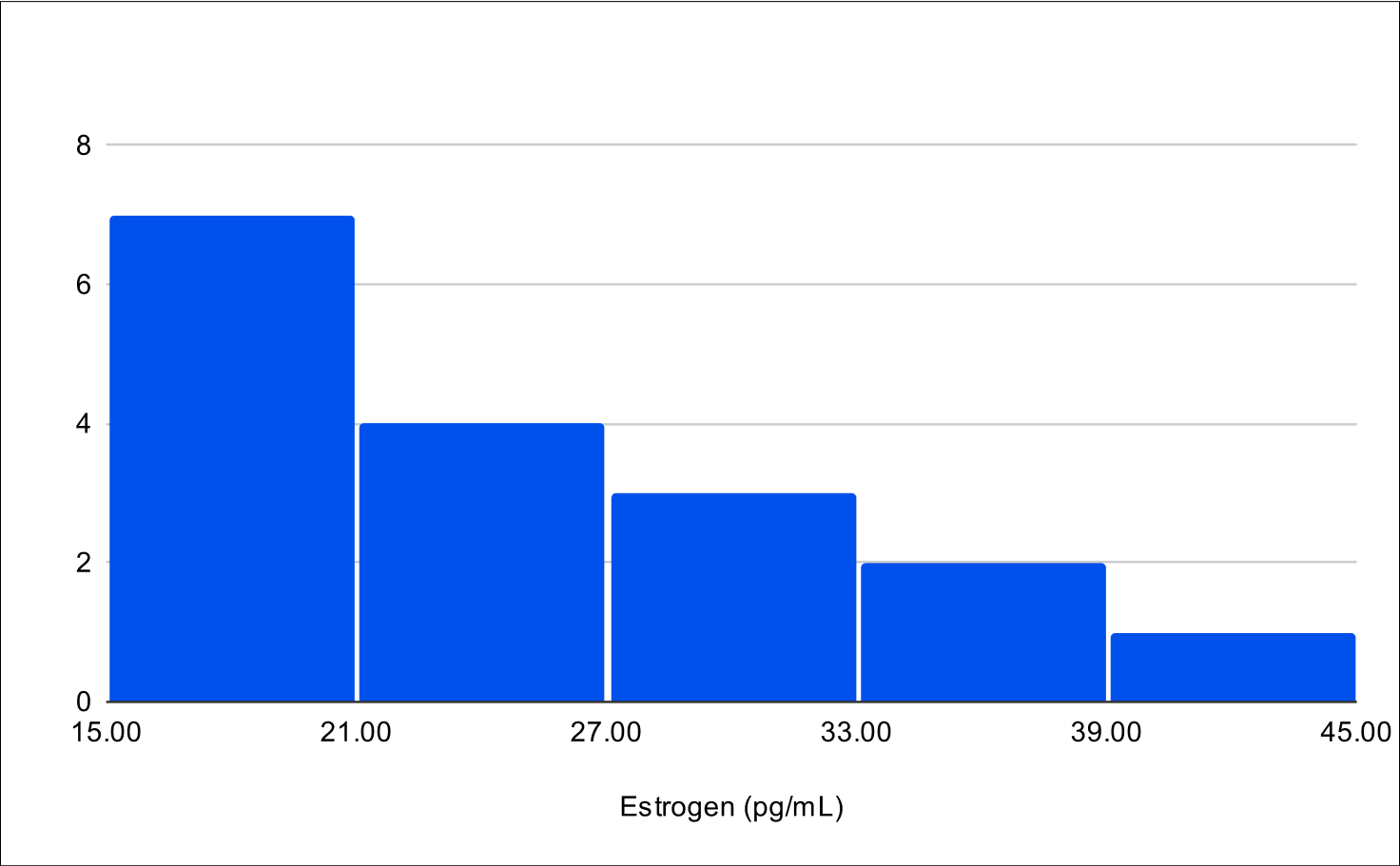
Baseline SHBG