White paper
Maximus’ Enclomiphene Protocol: A Safe and Effective Therapy to Restore Testosterone and Support Quality of Life in Men

S. Cameron Sepah, Ph.D.1,2; Hunter Henry, M.S.1; Gabriel Alizaidy, M.D., M.S.1; Nick Boerger, M.S.1
1. Maximus, Los Angeles, CA, United States
2. University of California, San Francisco, Department of Psychiatry, San Francisco, CA, United States
Corresponding Author:
S. Cameron Sepah, Ph.D.
Email: cam@maximustribe.com
Abstract
Men represent the population by age, gender, region, and race/ethnicity
23 Referenced studies
Purpose
Declining testosterone levels are observed in men with age and have also been observed generationally. Traditional treatment options for low testosterone, or hypogonadism, often have limitations and are often not provided for eugonadal men with testosterone levels within the normal range. This study was undertaken to evaluate the effectiveness of an online telemedicine treatment protocol, which includes enclomiphene citrate, for hypogonadal and eugonadal men seeking to optimize their health and performance.
Materials and Methods
1,250 male participants enrolled online in Maximus’ Testosterone Protocol, a telemedicine-delivered and doctor-prescribed treatment plan that included at-home blood testing, a prescription of enclomiphene citrate, and health coaching to improve health behaviors using community-based social accountability. Participants were assessed over time to monitor biomarkers (total and free testosterone, LH, and SHBG) and self-reported hypogonadal symptoms.
Results
Maximus’ Testosterone Protocol significantly increased free and total testosterone levels (89.7% and 81.8% relative increase at follow-up, respectively, p < 0.001), LH (87.1%, p < 0.001), and SHBG (12.4%, p < 0.001). In particular, there was a dose-dependent response up to 12.5mg, with the two most common dosages, 6.25 mg and 12.5 mg, resulting in 67.0% & 150.3% relative increases in Free Testosterone. A secondary analysis, which excluded 80 participants suspected of having primary hypogonadism or prior TRT use, revealed even more pronounced increases in free testosterone (96.5% increase, p < 0.001) and total testosterone (87.7% increase, p < 0.001). Most participants stayed on the protocol long-term (up to 3+ years) without adverse effects. Significant improvements were also observed in participant quality of life (qADAM) (79.9% with improvement) and depression/anxiety (PHQ-4) (67.1% with improvement).
Conclusions
On average, participants in Maximus’ Testosterone Protocol experienced a doubling of free testosterone levels, in addition to significant enhancements in holistic well-being, encompassing mood, libido, energy, athletic and work performance, erection quality, strength, and overall quality of life. The data further demonstrates the potential to deliver high-quality health care via telemedicine and in particular, enclomiphene citrate as a safe and effective long-term treatment for hypogonadal and eugonadal men seeking to optimize their health and performance.
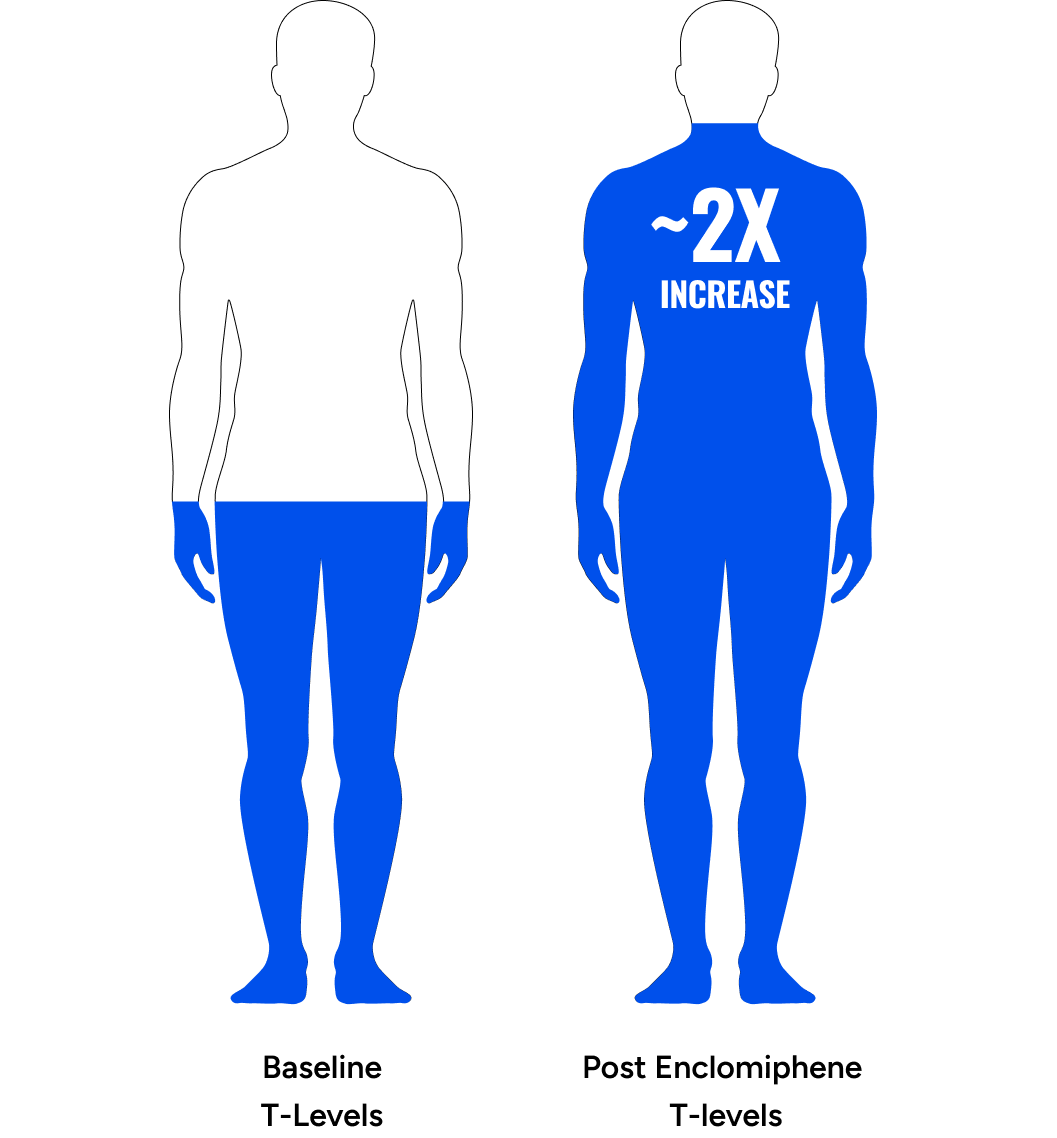
Background
Declining Testosterone
Average male total and free testosterone decreases with advancing age.1
These age-related decreases become noticeable among men in their 30s and 40s, even earlier for some. Obesity, chronic diseases such as type II diabetes, poor sleep, and infectious diseases are associated with lower testosterone at earlier ages.2 Other factors such as stress, poor nutrition, sedentary lifestyle, lack of sun exposure, and exposure to microplastics also impact testosterone negatively across all ages. As life expectancy increases, the average male is increasingly exposed to detrimental environmental and lifestyle factors. As such, lower testosterone levels are being observed at all ages across the world.
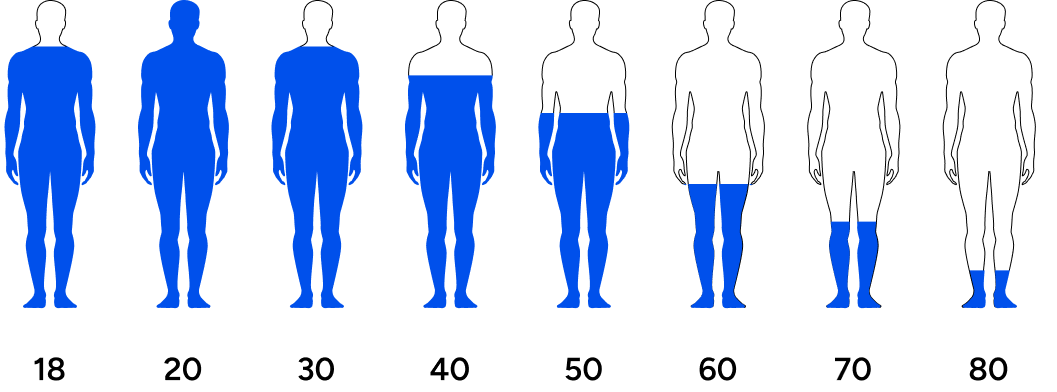
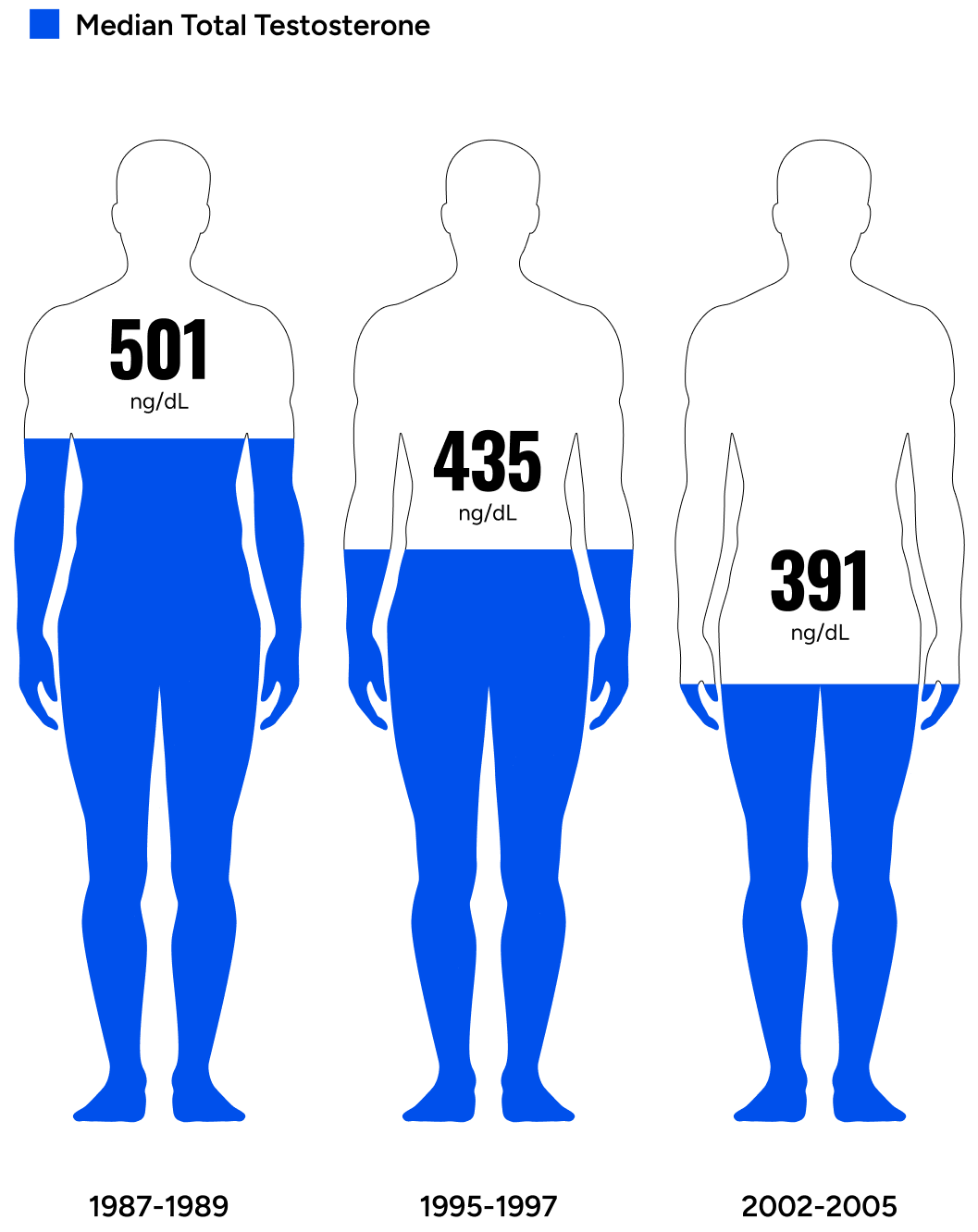
Men’s testosterone levels are decreasing across generations.
Travison et al. investigated testosterone levels in men across generations and concluded, “The past 20 yr have seen substantial age-independent decreases in male serum testosterone (T) concentrations, decreases that do not appear to be the consequence of the contemporaneous trends in health and lifestyle considered here.”3 The study asserted that regardless of lifestyle factors (the amount of exercise or quality of diet), men are experiencing a steady drop in testosterone levels over the last few decades.
This means that younger men have lower testosterone than their fathers or grandfathers did at their age. Symptoms of low testosterone include lack of motivation, lack of energy, low libido, poor life outlook, fat accumulation, increased risk for chronic conditions, and countless other undesirable outcomes.
Symptoms of low testosterone include lack of motivation, lack of energy, low libido, poor life outlook, fat accumulation, increased risk for chronic conditions, and countless other undesirable outcomes.
Low testosterone is defined by the American Urology Association as total testosterone less than or equal to 300 ng/dL.4 However, defining low testosterone solely by total testosterone may be misleading. participants may meet the standard definition of low testosterone but report no symptoms. Participants who receive treatment may achieve total testosterone levels within the normal range without reporting any symptomatic improvement.5 Therefore, treating hypogonadism on the basis of a number (lower than normal total testosterone) fails to address the complexity of hypogonadism.
As explained by Paduch et al, many assays used to define “normal” are developed in older participants and are not necessarily representative of the expected “normal” ranges in younger, healthy participants. It is further argued that measurements of free testosterone or bioavailable testosterone are preferred, because total testosterone levels are “equivocal or fail to reflect clinical presentation”.6 Simply put, men with eugonadal total testosterone may still present with hypogonadal symptoms, highlighting the importance of taking presenting symptoms into consideration along with lab validated biomarkers when evaluating and treating this population.
Options For Treating Hypogonadism
Testosterone replacement therapy (TRT) has been the standard treatment for participants with low testosterone levels for many years.6
Testosterone replacement therapy (TRT) has been the standard treatment for participants with low testosterone levels for many years.6 As the name suggests, TRT uses synthetic testosterone to replace the body’s depleted stores. TRT is available in many forms including intramuscular injection, transdermal patch, topical cream/gel, and oral/buccal delivery.
Some of the most common reasons TRT is prescribed are to increase libido, maintain muscle and decrease fat, maintain bone strength and function, and to enhance energy and cognition. Improvements in motivation, muscle and strength with TRT have made it a widely known synthetic performance-enhancing drug.
Many men who want to discontinue standard TRT struggle to do so because of withdrawal that results from the dependency created by TRT as a result of the shutdown in normal hypothalamic–pituitary–gonadal (HPG) axis function.7 Discontinuation of TRT can result in weight gain, low energy, low libido, irritability, and severe depression.
Stopping TRT can cause:
- weight gain
- low energy
- low sex drive
- irritability
- severe depression
Side Effects of trt:
- prostate cancer progression
- polycythemia
- peripheral edema
- cardiac and hepatic dysfunction
TRT is not the only treatment for low testosterone, as newer alternatives offer greater convenience and less adverse effects.
Enclomiphene citrate, an oral selective estrogen receptor modulator (SERM), works by increasing testosterone levels by priming the HPG axis to produce more endogenous native testosterone, rather than relying on exogenous testosterone, as with TRT.
Researchers compared FSH, LH, and testosterone levels in overweight males aged 18-60 years with low-normal testosterone (≤ 300 ng/dL) treated with enclomiphene or 1.62% Androgel, a topical form of TRT. Over 16 weeks, treatment with enclomiphene was associated with significantly increased LH, FSH and testosterone. Further, participants treated with enclomiphene had normal sperm concentration, while those treated with Androgel TRT had reduced sperm counts.8 The authors concluded that enclomiphene is a promising treatment option for participants who want to increase testosterone while conserving testicular function and fertility.9
Enclomiphene is derived from clomiphene citrate, an FDA-approved drug consisting of two stereoisomers, 62% enclomiphene and 38% zuclomiphene. Specifically, enclomiphene acts on the hypothalamus, blocking estrogen receptors, causing an increase in gonadotropin-releasing hormone (GnRH). This stimulates the pituitary gland to produce more luteinizing hormone (LH), and follicle-stimulating hormone (FSH), which stimulates the testes to produce more intratesticular testosterone and sperm.
Zuclomiphene improves testosterone production to a much lower degree,10 and it is postulated that this isomer is responsible for the unfavorable side effects of long-term clomiphene because zuclomiphene is an estrogen agonist (while enclomiphene is an estrogen antagonist). Furthermore, zuclomiphene has a half life of nearly 30 days, while enclomiphene has a half life of 10.5 hours, which causes zuclomiphene to build up in the system. In a study by Helo et.al., which utilized a 3:2 enclomiphene:zuclomiphene racemic mixture of clomiphene, prolonged treatment resulted in a 1:20 enclomiphene:zuclomiphene ratio of serum concentration.11 Therefore, usage of enclomiphene, without zuclomiphene, is understood to have a more predictable effect on testosterone production than clomiphene, with a lower risk of clomiphene-specific side effects.
Common off-label use of clomiphene includes treatment of male hypogonadism and infertility, but its estrogenic side effects can negatively impact sperm quality. Enclomiphene, being devoid of the estrogenic isomer, may potentially have a more favorable effect on testosterone and sperm quality. Other treatments for male hypogonadism not discussed in this paper include aromatase inhibitors (AI) and human chorionic gonadotropin (hCG).12
To date, research on enclomiphene has focused on participants with secondary hypogonadism as defined by total testosterone levels.810 The following study characterizes enclomiphene’s effectiveness at increasing testosterone and alleviating hypogonadal symptoms in a cohort of participants presenting diverse demographic and health statuses. Some participants met traditional hypogonadal definitions based on total testosterone at baseline, while others presented hypogonadal symptoms with “eugonadal” testosterone levels.
Participants and Methods
Study Population
All participants were males between 18 and 69 years of age who reported symptoms of hypogonadism in a baseline questionnaire.
For inclusion in this study, participants needed to have completed a baseline blood assessment (pre-treatment), at least one follow-up blood assessment (post-treatment), a baseline medical questionnaire (pre-treatment), and a follow-up medical questionnaire (post-treatment). The post-treatment blood sample was collected three to four weeks after treatment following dosage titration to ensure peak serum concentrations of enclomiphene were achieved.
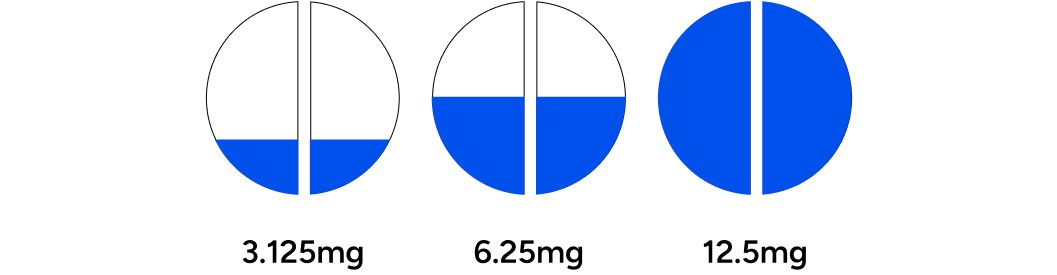
Participants began with a starting dosage of 3.125mg, 6.25mg, or 12.5mg. A board-certified physician determined the initial dosage for each individual based on an evaluation of their baseline blood test results and answers from the baseline questionnaire. Three to four weeks into treatment, the participant was assessed for symptom improvement and potential adverse effects. At this juncture, dosage could be increased (up to 25mg) or decreased. If there were changes to the dosage, participants were advised to postpone their post-treatment blood assessments until they had been on the new dosage for an additional three to four weeks. Any subsequent dose adjustments also warranted additional blood assessments in a similar manner, as required.
Exclusion criteria included self-reported recent or current use of testosterone replacement, anabolic steroids, selective androgen receptor modulators (SARMs), aromatase inhibitors, or selective estrogen receptor modulators. Other exclusion criteria included treatment contraindications such as: known allergies to SERMs, history of prostate cancer, and blood thinner usage. Participants were required to be 18 years or older and to be biologically male.
Participants with LH < 1.0 IU/L were excluded due to strong indication of one of the following:
Hypogonadotropic hypogonadism
This is a condition where there is inadequate production of LH and FSH from the pituitary gland, leading to decreased testosterone production. It can be caused by various factors, such as dysfunction of the hypothalamus or pituitary gland, genetic disorders, tumors, certain medications, stress, or other underlying medical conditions.
Testicular failure
Conditions that affect the testicles, such as trauma, infection, radiation or chemotherapy, certain medications, or genetic disorders, can result in decreased production of testosterone and subsequently low LH levels.
Hormonal therapy
Some hormonal therapies, such as androgen deprivation therapy (ADT) used in the treatment of prostate cancer, can suppress LH levels as part of the treatment strategy to reduce testosterone production.
The participants included in the study were required to have completed a follow up assessment after completing at least 4 weeks medication on the Maximus Testosterone protocol (enclomiphene treatment).
1511 men
between 19 and 75 years of age
Of the 1,511 participants, nine completed their second blood test assessment before starting the medication. Twelve lacked LH measurements from their initial blood assessment, while 15 were missing LH measurements from their follow-up blood test. From the remaining 1,475 participants, 1,428 recorded an initial LH of at least 1 IU/L.
To maintain the study's integrity, medication usage was self-reported by participants. Those revealing the use of medications known to alter steroid levels, such as TRT, Anabolic Steroids, Clomid, Aromatase Inhibitors, or other SERMs during the onboarding process, were excluded. After excluding 41 men who confirmed using steroid-inducing medications, 75 voluntary withdrawals due to adverse effects, and another 146 who hadn't completed the full medical questionnaire during onboarding, 1,241 participants remained.
Among the 75 participants who voluntarily withdrew due to side effects, 80 treatment emergent adverse events (TEAEs) were observed. Importantly, there were no serious adverse events. The majority of these side effects were non-specific, with the most common being weight gain, changes in libido, allergic reactions, and acne. These findings are consistent with those reported in previous Phase 2 and 3 trials.13 Only one participant reported ocular changes, which may be influenced by their prior history of HCG, TRT, and clomiphene use. For a detailed breakdown of all TEAEs, please refer to Table A4 in the appendix.
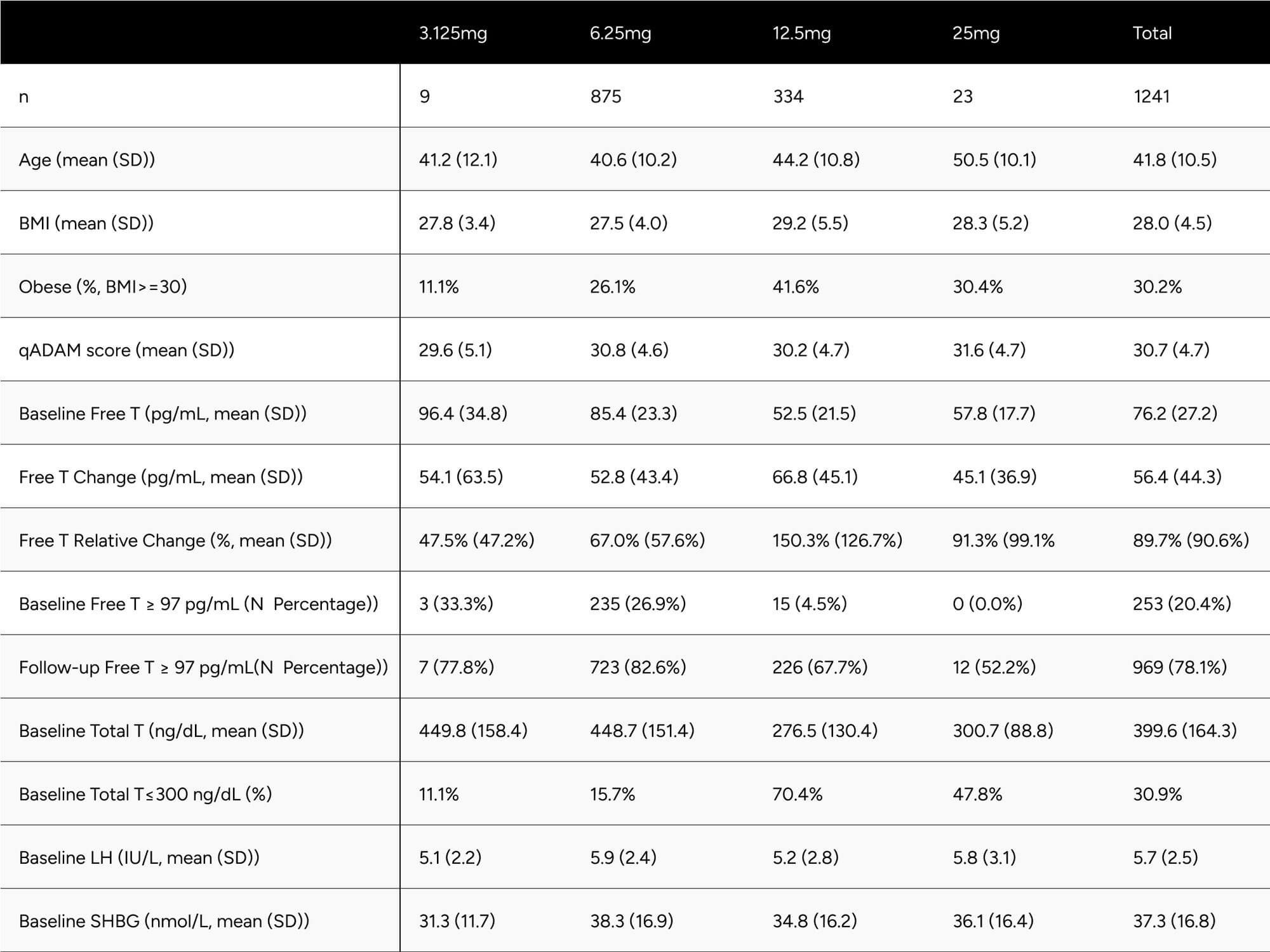
Table 1 presents descriptive statistics of the study population, including age, self-reported BMI, qADAM score, and baseline biomarkers. These statistics are further differentiated by the dosage participants were prescribed at the time of their latest follow-up blood test. Notably, only 9 and 23 participants were prescribed 3.125mg and 25mg dosages, respectively. When comparing participants on 6.25mg to those on 12.5mg, the latter were found to be older (44.2 vs. 40.6 years), had a higher BMI (29.2 vs. 27.5), and recorded a lower baseline free testosterone level (52.5 vs 85.4 pg/mL). Intriguingly, qADAM scores were nearly identical (30.2 vs 30.8).
Table 1: Study Population Descriptive Statistics
Data Collection
Participant demographics, health status (biomarker and self-reported), and treatment data were self-reported via online medical questionnaires, CLIA-certified at-home assessments using a capillary blood collection device, and an electronic medical record system.
Medical Questionnaire
The medical questionnaires were completed at baseline (pre-treatment) and follow-up (three to four weeks into treatment) through online self-reporting by participants via the Maximus application.
The questionnaires assessed many factors known to contribute to hypogonadism, including lifestyle behaviors, chronic conditions, quantitative Androgen Deficiency in Aging Males questionnaire (qADAM), Patient Health Questionnaire-4, height, weight, body fat, current medications, allergies, medical history, and family history. For a detailed view of the questionnaires used in this study, please refer to Supplementary Material 1.
Lab Testing
Samples were processed by a CLIA-certified, high-complexity clinical laboratory. Panels may vary slightly between participants but necessarily included:
Labs included:
- Total Testosterone (TT)
- Sex Hormone Binding Globulin (SHBG)
- Luteinizing Hormone (LH)
Free Testosterone (FT) was calculated using the Vermeulen equation, employing measured TT and SHBG, with an assumed albumin level of 4.3 g/dL. This method of calculation has been demonstrated to be a rapid, simple, and reliable index of bioavailable testosterone, comparable to Albumin-bound and Free Testosterone Concentration, and is suitable for clinical routine.14
Physicians requested follow-up lab testing be completed typically 3-4 weeks after dosage titration to assess the impact of treatment on hormone levels.
Statistical Analysis
While the sample size was quite large, we tested the data for normality using the Shapiro-Wilk Test. With a null hypothesis of the data being normally distributed, there was sufficient evidence to reject the null hypothesis in favor of the data being not normally distributed. The lab results had a positive skew, thus we used the non-parametric Wilcoxon Signed-Rank test. To determine the symptom improvement before and after treatment, we used the McNemar’s Test.
In addition to the AUA’s standard definition for hypogonadism (Total Testosterone < 300 ng/dL), we benchmarked FT and TT levels against reference ranges representing healthy nonobese 19-40 year old males, as collected in the Framingham Heart Study.15 The benchmarks used from the Framingham Heart Study data were 97 pg/mL for FT and 503 ng/dL for TT, representing -1 SD of Mean for Healthy, non-obese 19-40 year old men.
This benchmark was evaluated as it represents target testosterone levels for men looking to feel and perform as they did in their youth and in good health.
Results
Biomarker Changes
After treatment with enclomiphene, participants had significantly increased free and total testosterone levels.
56.4
Free Testosterone (ft)
FT was increased 56.4 pg/mL on average (from 76.2 pg/mL to 132.6 pg/mL), which translates to a 74.0% increase on average for participants (p < 0.001) (Table 2). At baseline, only 24.6% of participants were above -1 standard deviation of the average FT for healthy, non-obese 19-40 year olds. After treatment, 73.5% achieved this benchmark (increase of 48.9%). We observed 64.5% of participants achieved a 50% increase in FT from baseline (Table 3).
266.9
Total Testosterone (TT)
TT increased by 266.9 ng/dL on average (from 399.6 ng/dL to 666.49 ng/dL), which translates to an 66.8% increase on average for participants (p < 0.001) (Table 2). At baseline, only 20.4% of participants were above -1 standard deviation of the average TT for healthy, non-obese 19-40 year olds. After treatment, 78.1% achieved this benchmark (increase of 57.7%). At baseline, 69.3% of participants were above the standard eugonadal definition of 300 ng/dL TT, after treatment 96.1% (p < 0.001). We observed 59.4% of participants achieved a 50% increase in TT from baseline (Table 3).
4.0
Luteinizing hormone (lh)
LH was increased by 4.0 points on average, from 5.7 ng/dL to 9.7 ng/dL (p < 0.001). SHBG increased by 3.5 points, from 37.3 nmol/L to 40.8 nmol/L (p < 0.001) (Table 2).
Despite significant increases in SHBG (12.4%), TT increased in such a manner (81.8%) that it “outpaced” SHBG and participants experienced disproportionate increases in FT (89.7%).
In addition to the primary results, a secondary analysis was conducted that excluded 80 participants who demonstrated a decline in free testosterone levels during treatment. Such a decrease is unexpected given the therapeutic intent of enclomiphene to elevate testosterone levels. This unexpected decline raises concerns about potential factors that might skew the interpretation of the drug's efficacy.
It is hypothesized that these participants had undiagnosed primary hypogonadism or did not accurately report their ongoing and unreported TRT regimen during their baseline test.
Primary hypogonadism refers to an intrinsic malfunction of the testes which hinders their ability to produce adequate testosterone, irrespective of the signals received from the pituitary gland. Enclomiphene's mechanism of action involves the stimulation of the pituitary gland to enhance the secretion of LH.16 This, in turn, signals the testes to increase testosterone production. In cases of primary hypogonadism, the testes may not respond to this increased LH secretion, potentially leading to stagnant or even decreased testosterone levels.
On the other hand, men undergoing TRT receive exogenous testosterone. While this treatment can lead to an immediate boost in total testosterone levels, it can concurrently suppress the body's natural testosterone production. This suppression arises due to the body's recognition of the external testosterone source, whether it be short-acting, intermediate-acting, or long-acting testosterone. Each type will lead to inhibited LH secretion, albeit with varying degrees of suppression.17 If a participant either discontinues or inconsistently adheres to TRT, especially when such TRT usage is unreported, a rapid drop in testosterone levels may ensue. Introducing enclomiphene under these conditions might mask its intended effects due to the preceding decrease in endogenous testosterone from the unreported TRT.
Given these considerations, excluding participants with primary hypogonadism or those on unreported TRT is crucial to ascertain a more accurate representation of enclomiphene's efficacy.
Upon exclusion of these outliers, the results of the secondary analysis revealed a more pronounced outcome. FT experienced a significant increase, transitioning from a baseline of 75.0 pg/mL to 136.1 pg/mL, which corresponds to a rise of 96.5% (Table A1a). This change in FT markedly exceeds the elevation observed in the primary analysis, emphasizing the importance of filtering out potential non-responders or confounding factors.
Similarly, TT observed an upsurge of 87.7%, contrasting with the 81.8% recorded in the primary findings. Additional parameters such as SHBG and LH also evidenced elevated increments of 12.6% and 91.0%, respectively.
Further statistical details related to this secondary analysis are available in Table A1a in the appendix.
Table 2: Average Changes in Hormones Between Baseline & Follow-up
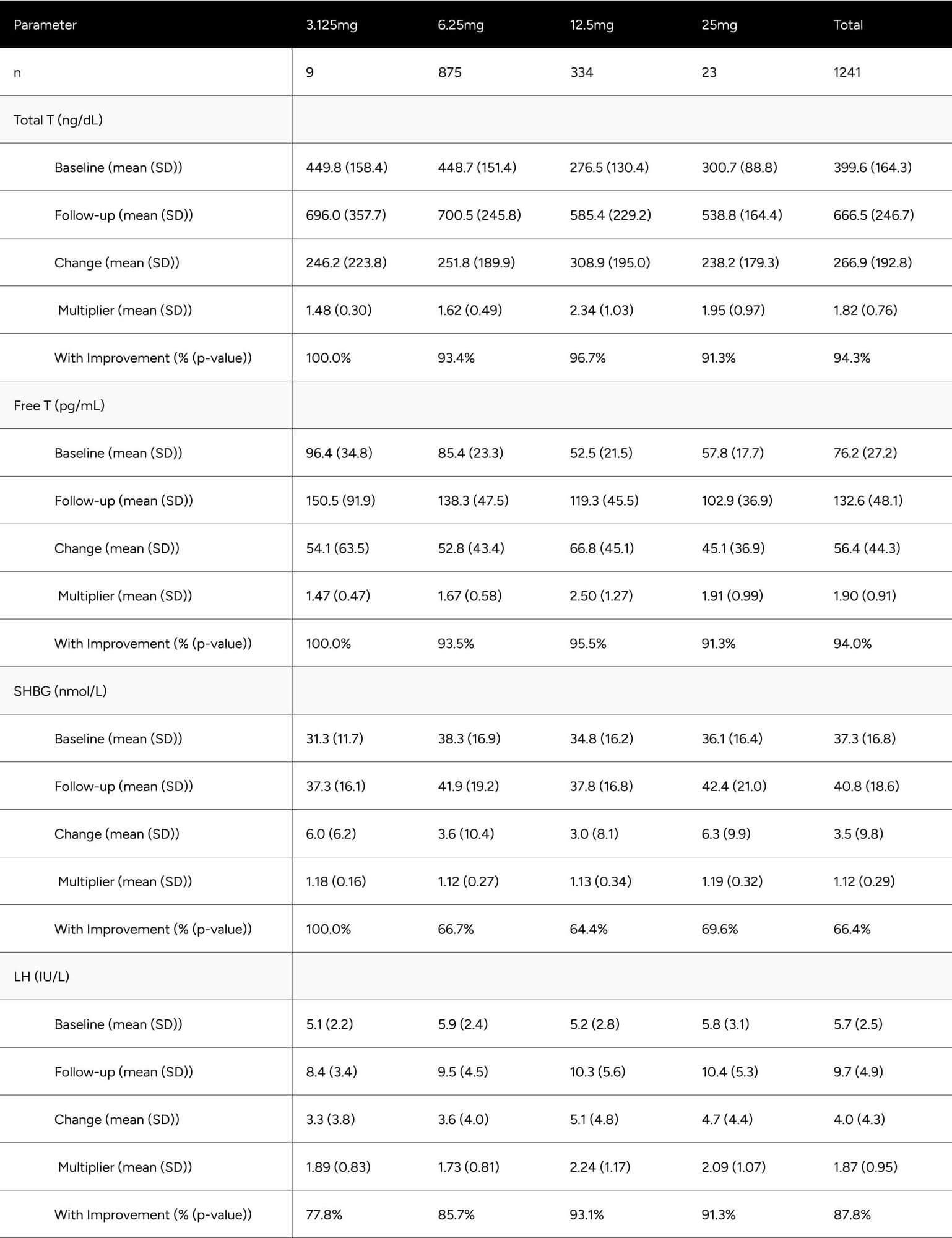
Table 3: Categorical Improvements in FT and TT
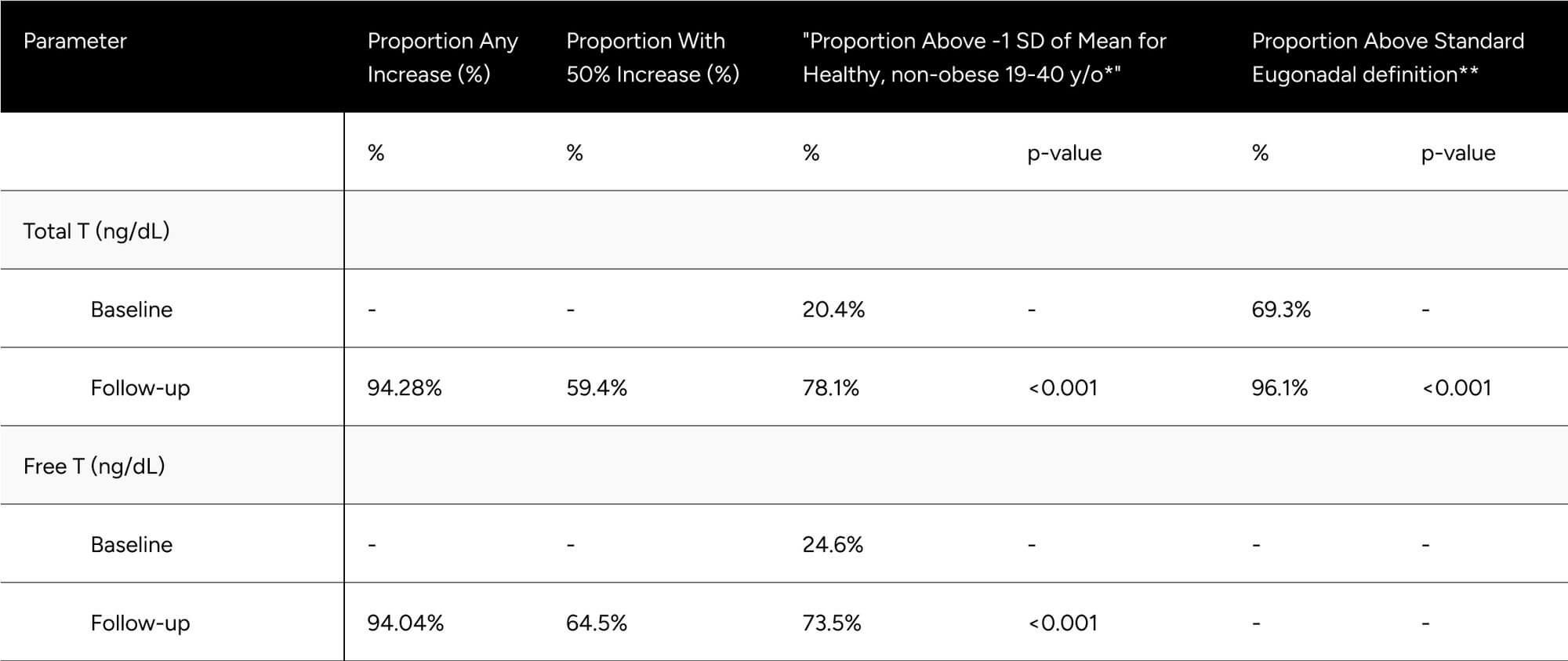
* Defined as 503 ng/dL for Total T, 97 pg/mL for Free T
** Defined as 300 ng/dL Total T. No definition set for Free T
We evaluated enclomiphene’s impact on hormone levels for “mixed hypogonadal” and healthy/”eugonadal” participants independently. The definition for mixed hypogonadism used was LH over 8.7 ng/dL and FT between 65 and 97 pg/mL at baseline. The threshold of 8.7 ng/dL for LH was chosen as it slightly exceeds the upper limit of the normal range for men over 18 years of age, which is around 1.8 to 8.6 IU/L according to Mount Sinai's guidelines.18 The lower FT limit of 65 pg/mL aligns with recommendations from the ISA, ISSAM, EAU, EAA, and ASA, which consider values under 65 pg/mL as below the normal lower limit for calculated free testosterone.19 The upper FT limit of 97 pg/mL is derived from the Framingham Heart Study, representing -1 SD of the mean for healthy, non-obese 19-40-year-old males.17
A participant exhibiting an LH level beyond the 8.6 IU/L threshold, coupled with FT in the 65-97 pg/mL, would typically be considered mixed hypogonadal, indicative of a potential testosterone production deficiency. Low testosterone can also arise from mixed hypogonadism, a condition that reflects defects not only in the testes but also in the hypothalamus and/or the pituitary. This mixed condition can be found in men with various conditions such as sickle-cell disease, thalassemia, alcoholism, glucocorticoid treatment, and in older men.19 While the term 'mixed hypogonadism' isn't frequently used in US clinical practice, it is generally subsumed under secondary hypogonadism and thus, these individuals were included in the primary and secondary analysis. In our findings, the FT increase was observed to be 76.1% for the mixed hypogonadism group, compared to the 89.7% in the primary analysis and 96.5% increase in the secondary analysis (Table A3). It is worth noting that the mixed nature results in differential presentations and responses to treatment.
Symptom Changes
Improvements at follow-up were seen at the highest rates for energy, sports ability, libido, work performance, life enjoyment, and happiness, with those seeing improvements at 55.1%, 50.3%, 48.3%, 46.9%, 43.0%, 42.6%. Erections, strength and endurance, and fall asleep after dinner in 40.3%, 38.1%, 29.1%. Despite the variance in rate of improvement across the questions, all were statistically significant (p < 0.001) (Table 4).
After treatment, all qADAM questions showed the proportion of participants with normal function at or above 80% aside from libido (73.7%). The proportion of participants with normal function was statistically significantly higher for all questions at follow-up (p < 0.001 for all) (Table 4).
The composite qADAM score improved in 79.9% of participants (p < 0.001), with normal function increasing from 60.5% to 84.9% (p < 0.001) (Table 4).
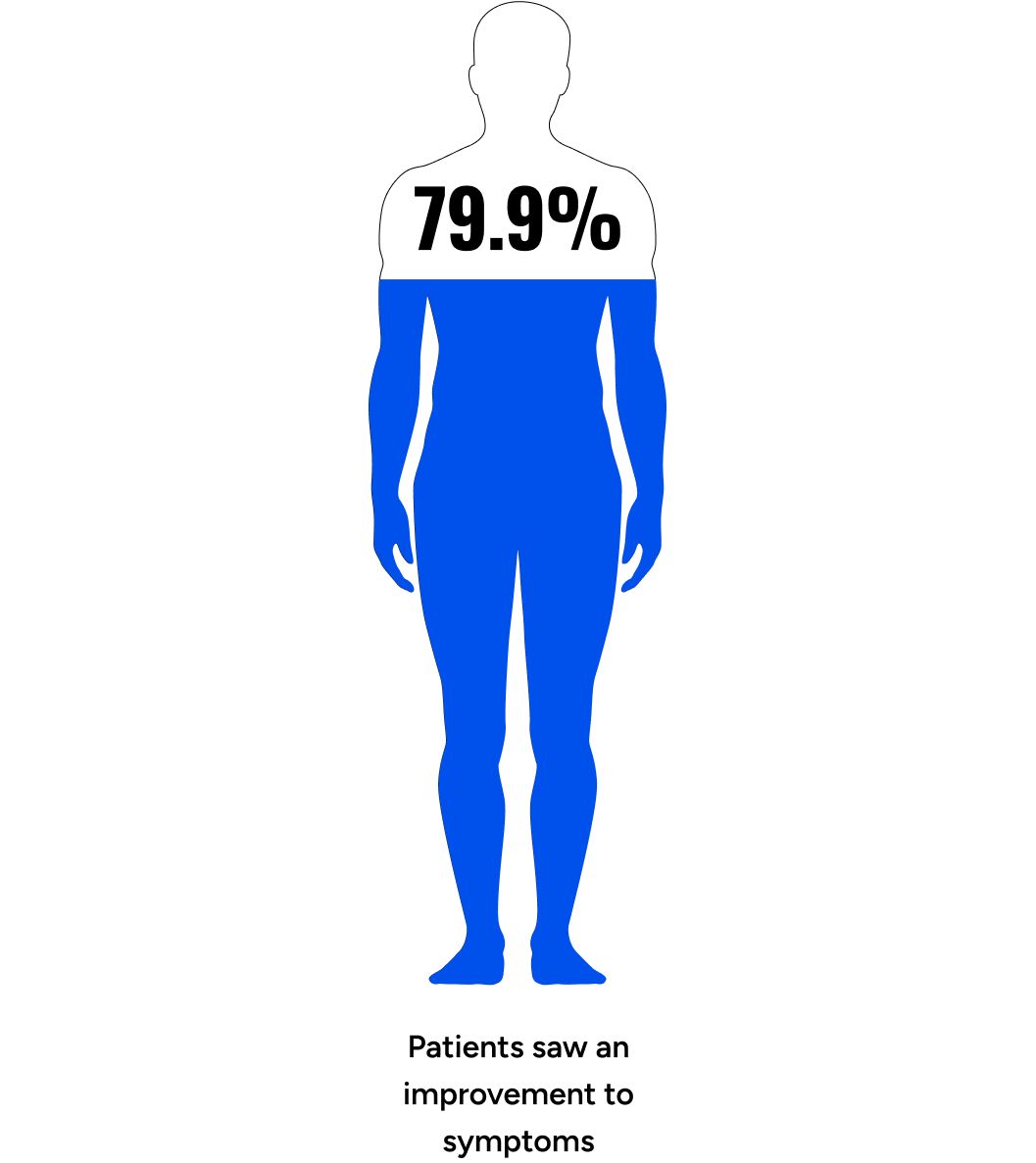
Table 4: Improvements in qADAM Scores
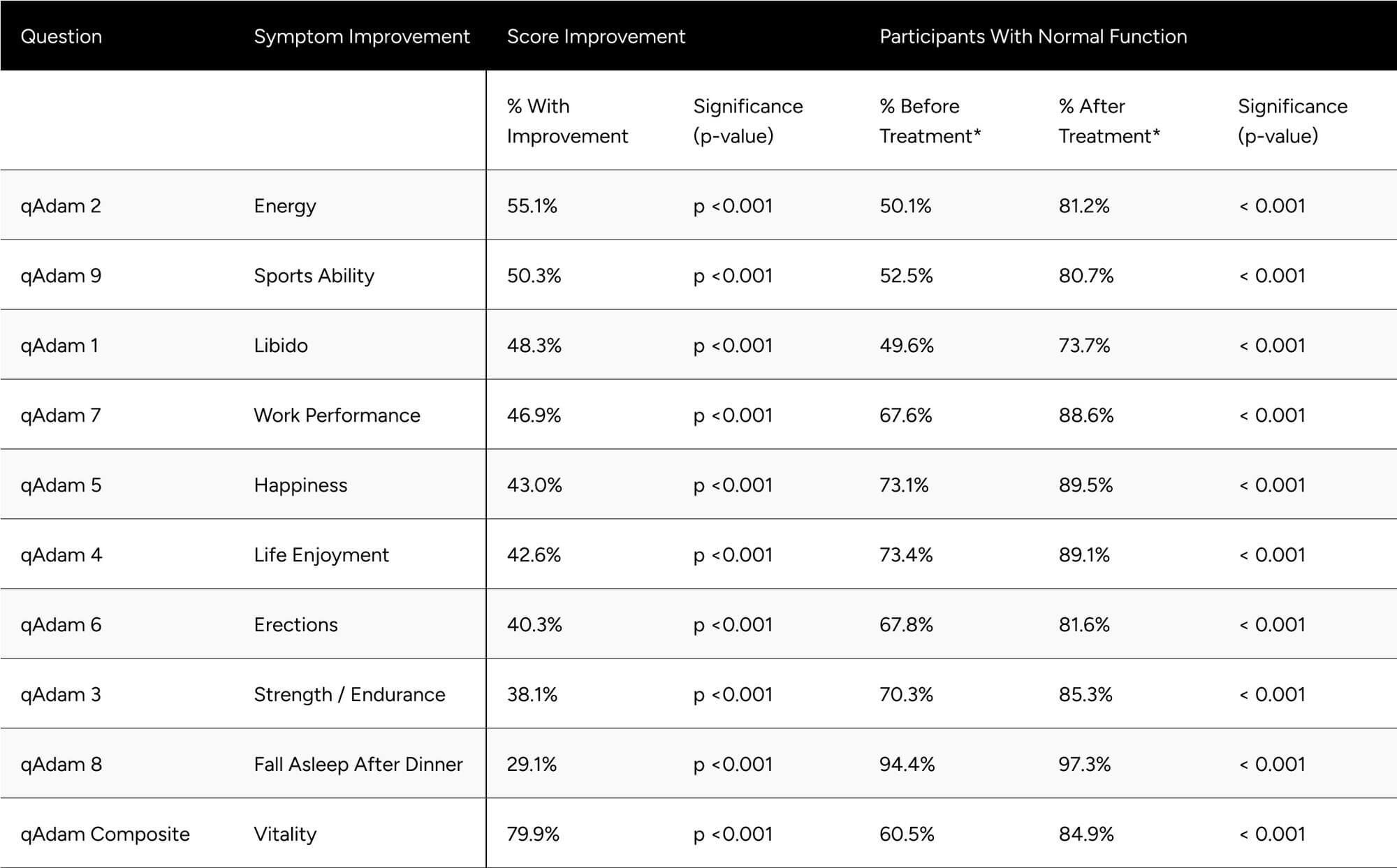
* Normal function is defined as a score of at least 3 for individual questions and average of 30 for the composite
Evaluating Patient Health Questionnaire-4 (PHQ-4) components, 54.6% of respondents showed improvement in anxiety (p < 0.001), going from 65.5% to 86.4% with normal function (p < 0.001). We observed that 54.3% of respondents showed improvement in the depression score (p < 0.001), going from 70.8% to 90.0% with normal function (p < 0.001). Combining anxiety and depression components, we saw that 67.1% of participants experienced a net improvement in mood (p < 0.001) (Table 5).
The percentage of participants with normal function (PHQ-4 combined score) nearly doubled from 29.7% at baseline to 56.2% at follow-up (p < 0.001). At follow-up, 91.5% of participants showed normal or mild depression/anxiety, up from 70.8% at baseline (p < 0.001) (Table 5).
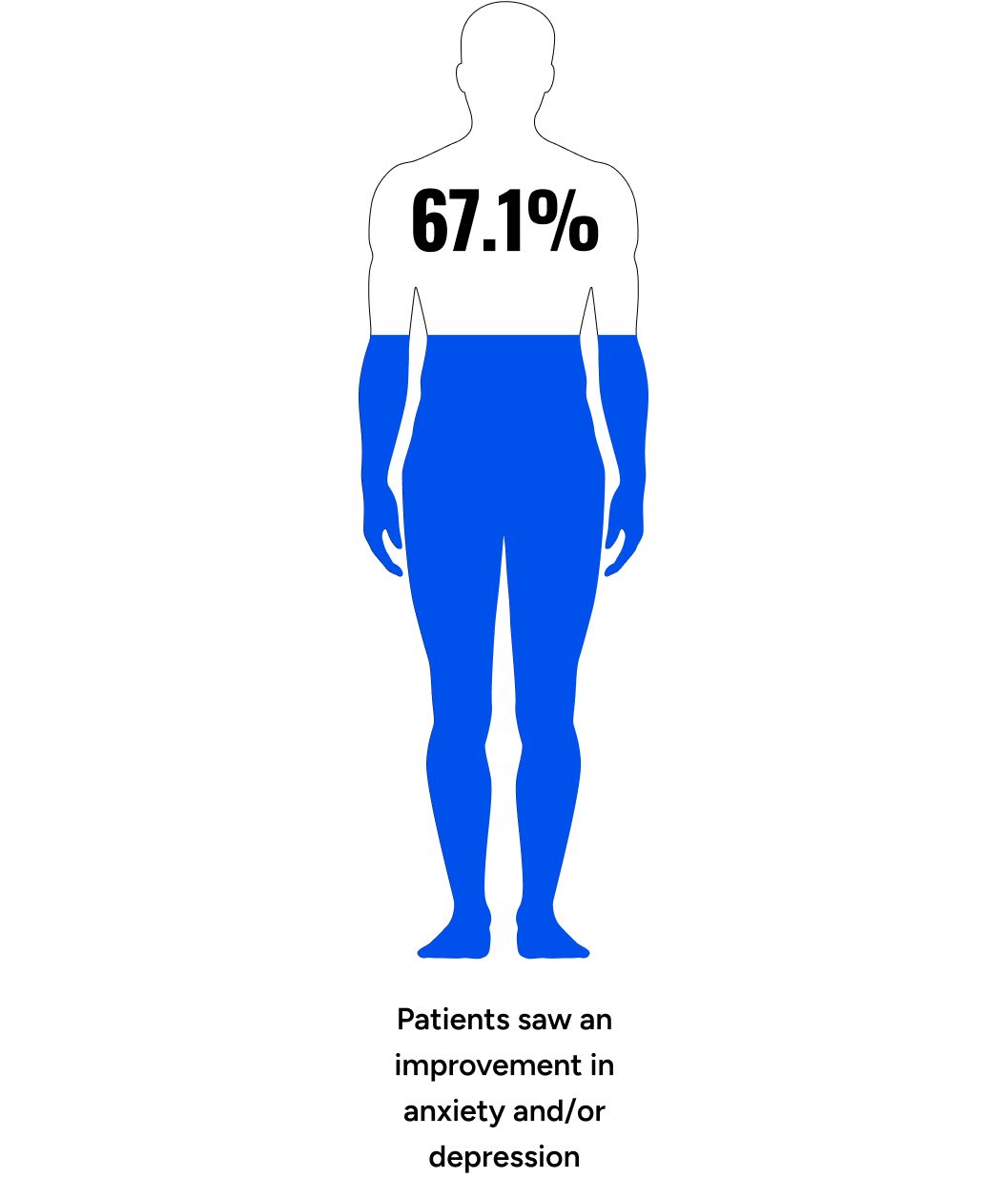
Table 5: Improvements in PHQ-4 Scores
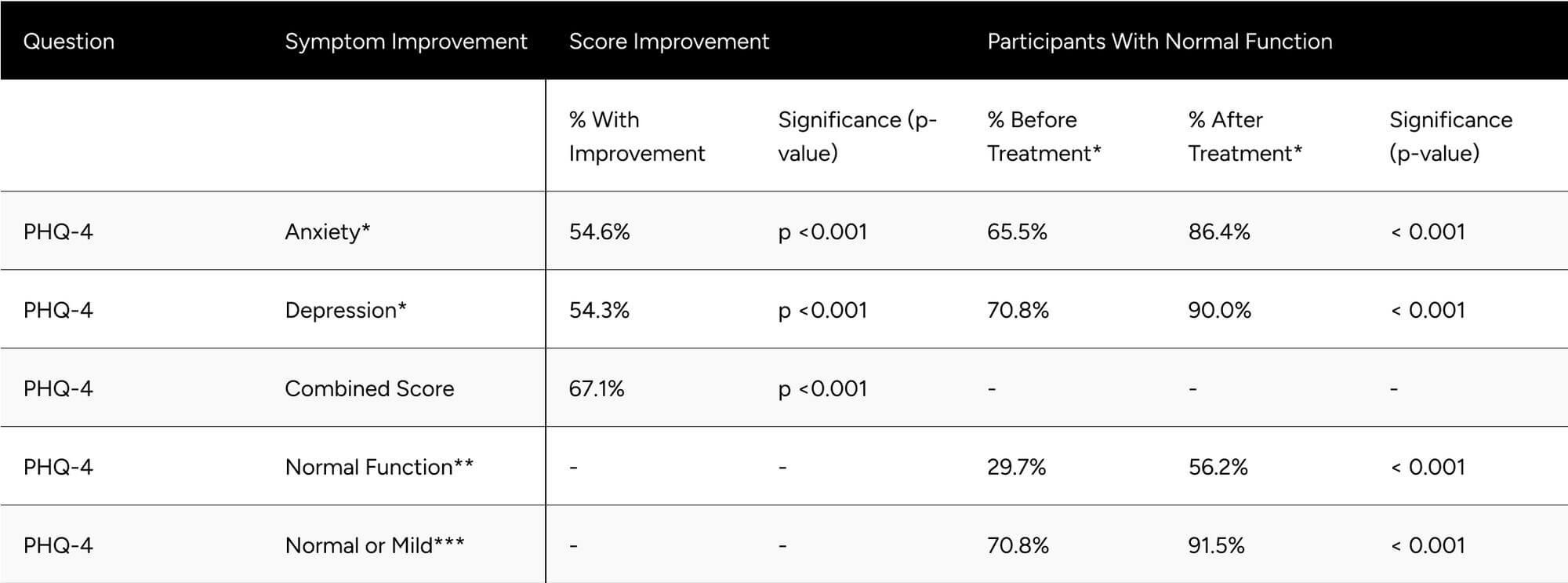
* Normal function PHQ-4 Anxiety and Depression is a score of less than 3.
** Normal function for the PHQ-4 combined score is a score of less than 3.
** Normal or mild function for PHQ-4 combined score is a score of less than 6.
Discussion
Findings
Hypogonadal and eugonadal males both respond overwhelmingly positively to treatment with enclomiphene, with 94% achieving some increase in testosterone and 80% achieving some increase in testosterone-related symptoms/quality of life.
Following treatment with enclomiphene, nearly every participant in the study surpassed the American Urology Association eugonadal threshold of above 300 ng/dL total testosterone. However, a total testosterone of 300 ng/dL is between the 1st and 2.5th percentile for healthy, non-obese males aged 19-40 years olds. It is reasonable to conjecture that the vast majority of men would aspire to have the testosterone levels of a healthy, young man. As such, in our practice we also evaluate participants against benchmarks set on that population.
Only 20.4% of participants were above the threshold of being within one standard deviation of total testosterone for healthy, young men at baseline. Comparatively, 69.3% of participants met the 300 ng/dL threshold presented by the AUA at baseline. Considering that participants in this study were seeking treatment for hypogonadal symptoms, we believe the threshold set by the AUA may be too conservative and should be used in conjunction with participant symptoms and other benchmarks. With treatment, the percentage of participants above the mean total testosterone minus one standard deviation improved to 78.1%.
Every qADAM question (besides loss of height) saw significant improvements in the study population. These questions were nearly all at 80% or higher “normal” function after treatment. The question that saw the greatest increase in proportion of “normal” function after treatment was energy, which has improved from 50.1% to 81.2% of participants with normal function. Libido is complex and multifactorial.20 Nevertheless, 48.3% of participants experienced improvements and the proportion with normal function increased from 49.6% to 73.7%. Future research may explore modulators of enclomiphene’s ability to improve libido, especially given its mechanism modulating estrogen, which is essential for libido in men.21 Overall, 84.9% participants achieved normal levels of testosterone-related symptoms/quality of life.
This study demonstrates that enclomiphene is an effective and relatively safe, and thus often preferable, treatment option for male participants experiencing symptoms of hypogonadism.
Compared to traditional TRT, enclomiphene is far more convenient and, as validated in this study, non-suppressive of luteinizing hormone and thus testicular function. This study also demonstrates that participants that do not meet the traditional definition of hypogonadism may benefit from treatment as well.
Limitations
Given that participants were self-selected into treatment they paid for out-of-pocket, there's a potential selection bias in the study population towards men who responded favorably and thus remained as participants long enough to complete at least one follow-up lab.
Another potential limitation arises from the exclusion criteria of our study, which might have been conservative. Specifically, the primary results may not have accounted for underlying primary hypogonadism or inaccurate reporting of ongoing TRT regimens. Primary hypogonadism involves a dysfunction within the testes that prevents them from producing adequate testosterone levels. As enclomiphene stimulates the pituitary gland to enhance LH secretion, which signals the testes to boost testosterone production, this treatment may not be effective in those with primary hypogonadism.
Additionally, men undergoing TRT receive external testosterone, which can suppress natural testosterone production. If enclomiphene is introduced after the discontinuation or inconsistent use of TRT, its effects could be masked by the drop in endogenous testosterone levels due to the TRT.
An accurate representation of enclomiphene's efficacy demands the exclusion of participants with primary hypogonadism or those on TRT. This became even more evident in our secondary analysis. Upon the exclusion of these outliers, results showcased a more pronounced positive outcome in FT, TT, SHBG, and LH levels.
Notably, other studies on enclomiphene, such as the Repros study, were more deliberate in their exclusion criteria, specifying restrictions such as "a recent history of alcoholism or illegal substance or steroid abuse (< 2 years)" and "any prior use of testosterone treatments (injectable, pelleted, transdermal or sublingual) within the last 6 months".10 Our study could have benefited from incorporating similar restrictions for a more refined participant selection.
Reference
Appendix
Figure A1
Initial LH Influence on Free-T Response (%)
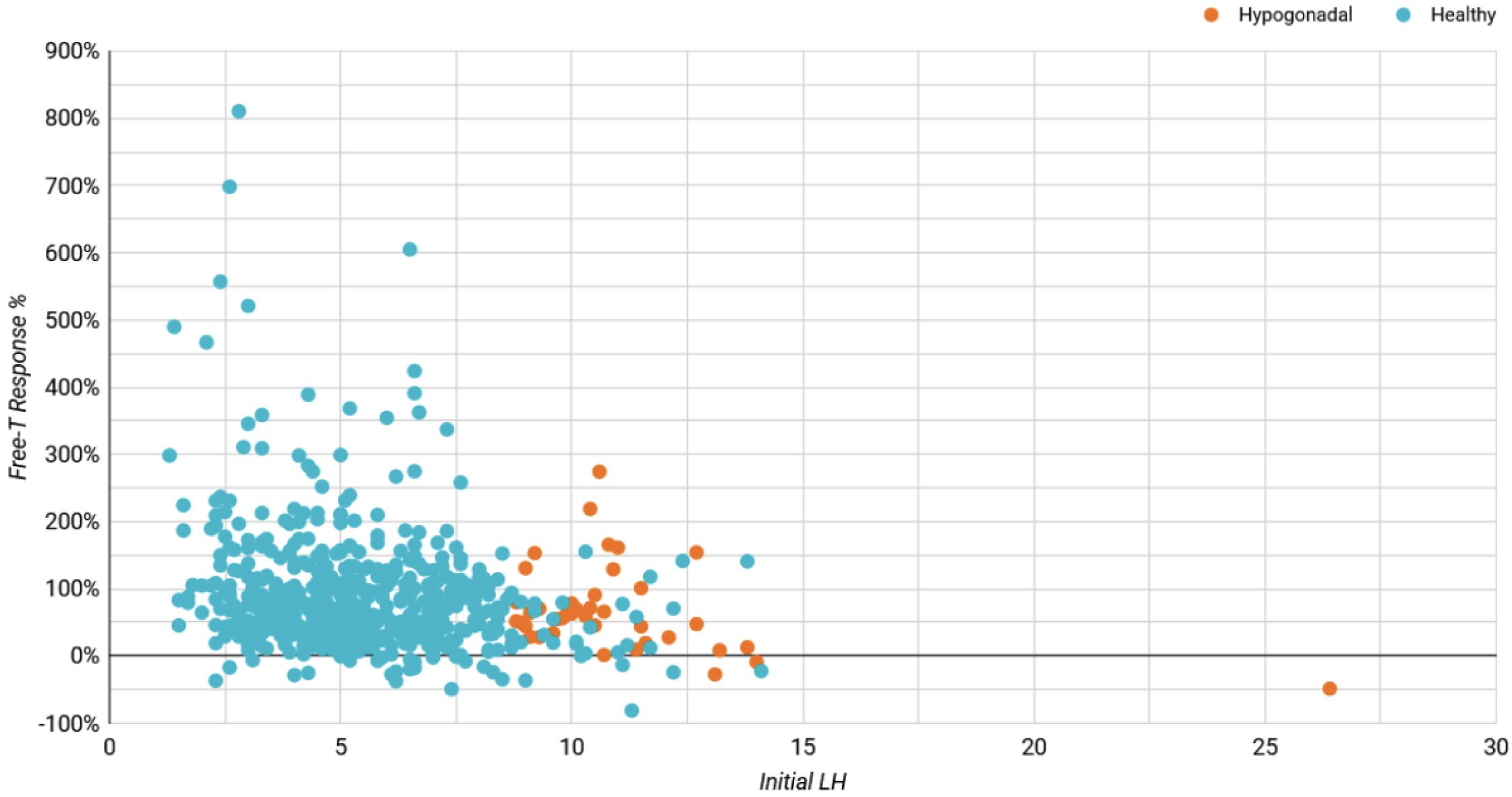
Table A1a: Expected Change in Hormones Between Baseline & Follow-up for Non-Confounded, Non-Primary Hypogonadal Men
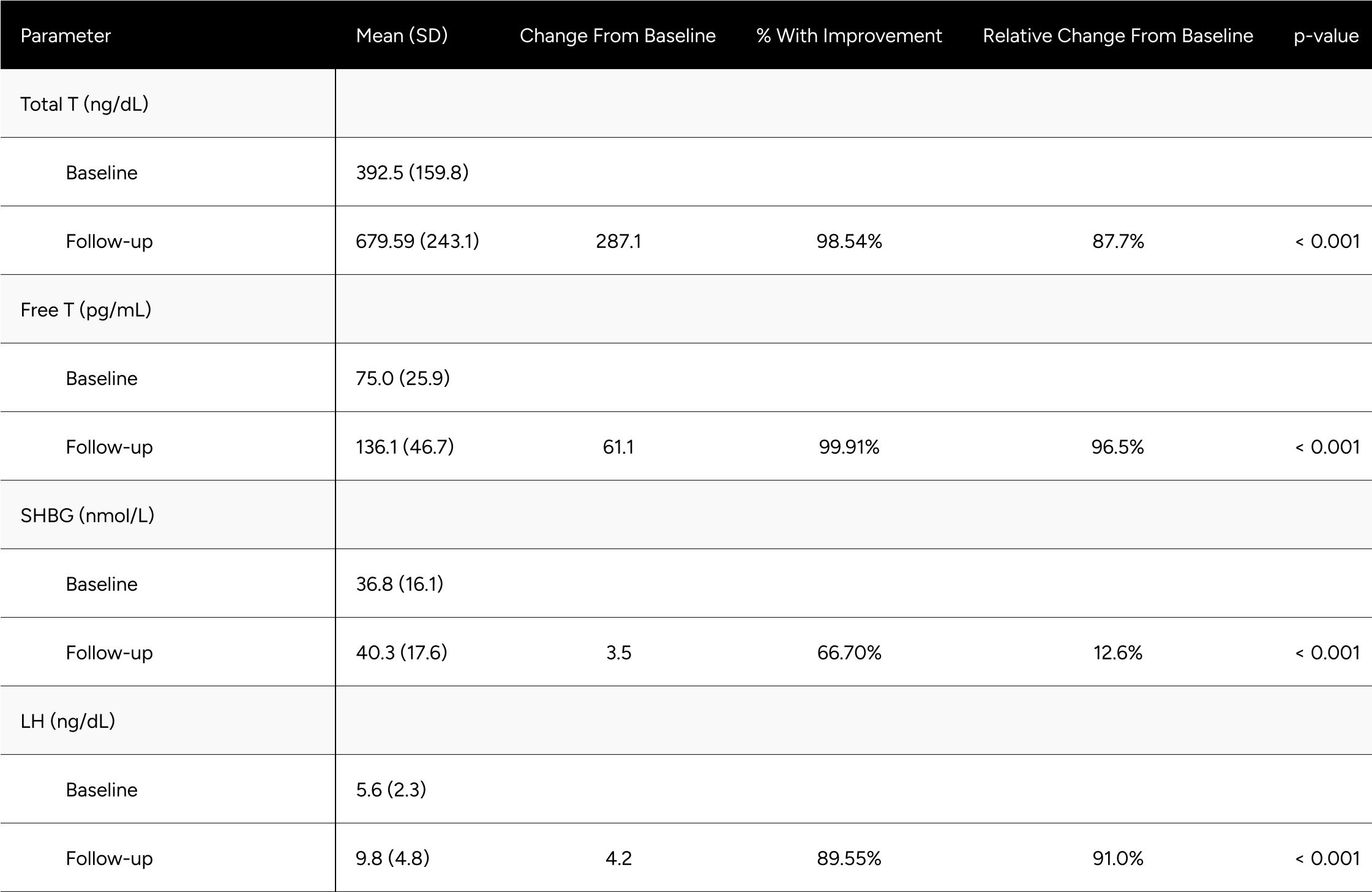
Table A1b: Change in Hormones Between Baseline & Follow-up for Non-Confounded, Non-Primary Hypogonadal Men
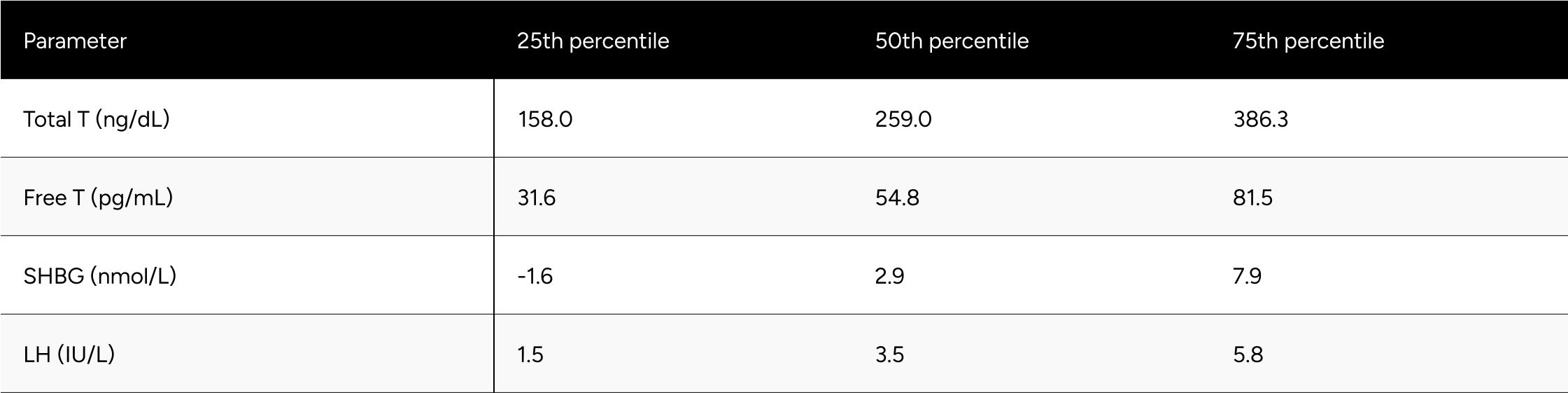
* Men without a decrease in Free-Testosterone.
* 80 participants who demonstrated a decline in testosterone levels during treatment .It is hypothesized that these participants had undiagnosed primary hypogonadism or did not accurately report their ongoing TRT regimen during their baseline test.
Table A1c: Percentage Change in Hormones Between Baseline & Follow-up for Non-Confounded, Non-Primary Hypogonadal Men
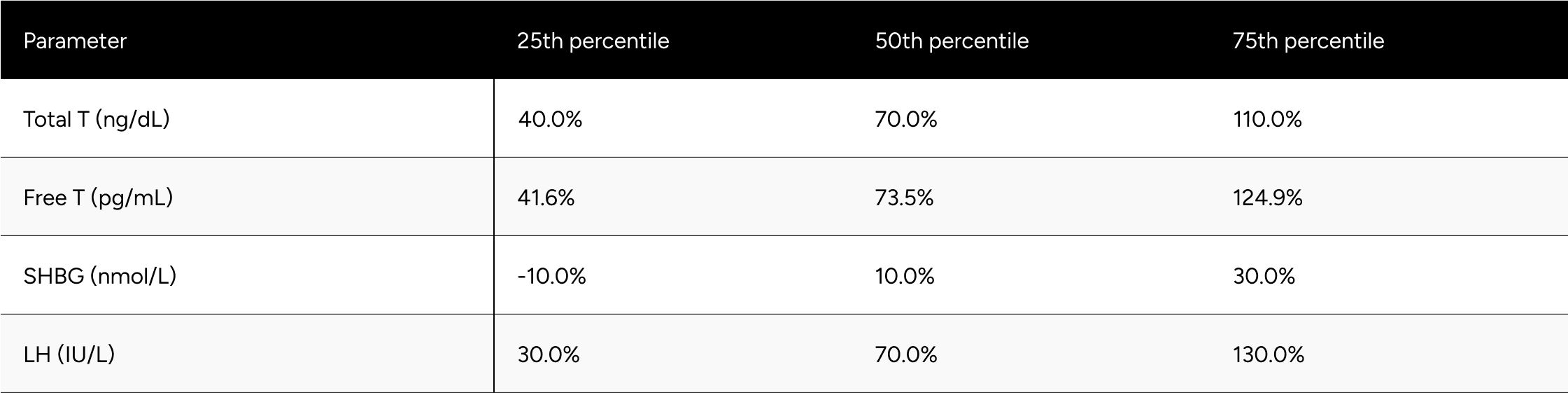
* Men without a decrease in Free-Testosterone.
* 80 participants who demonstrated a decline in testosterone levels during treatment .It is hypothesized that these participants had undiagnosed primary hypogonadism or did not accurately report their ongoing TRT regimen during their baseline test.
Table A2: Average Changes in Hormones Between Baseline & Follow-up For *Mixed Hypogonadal* Men
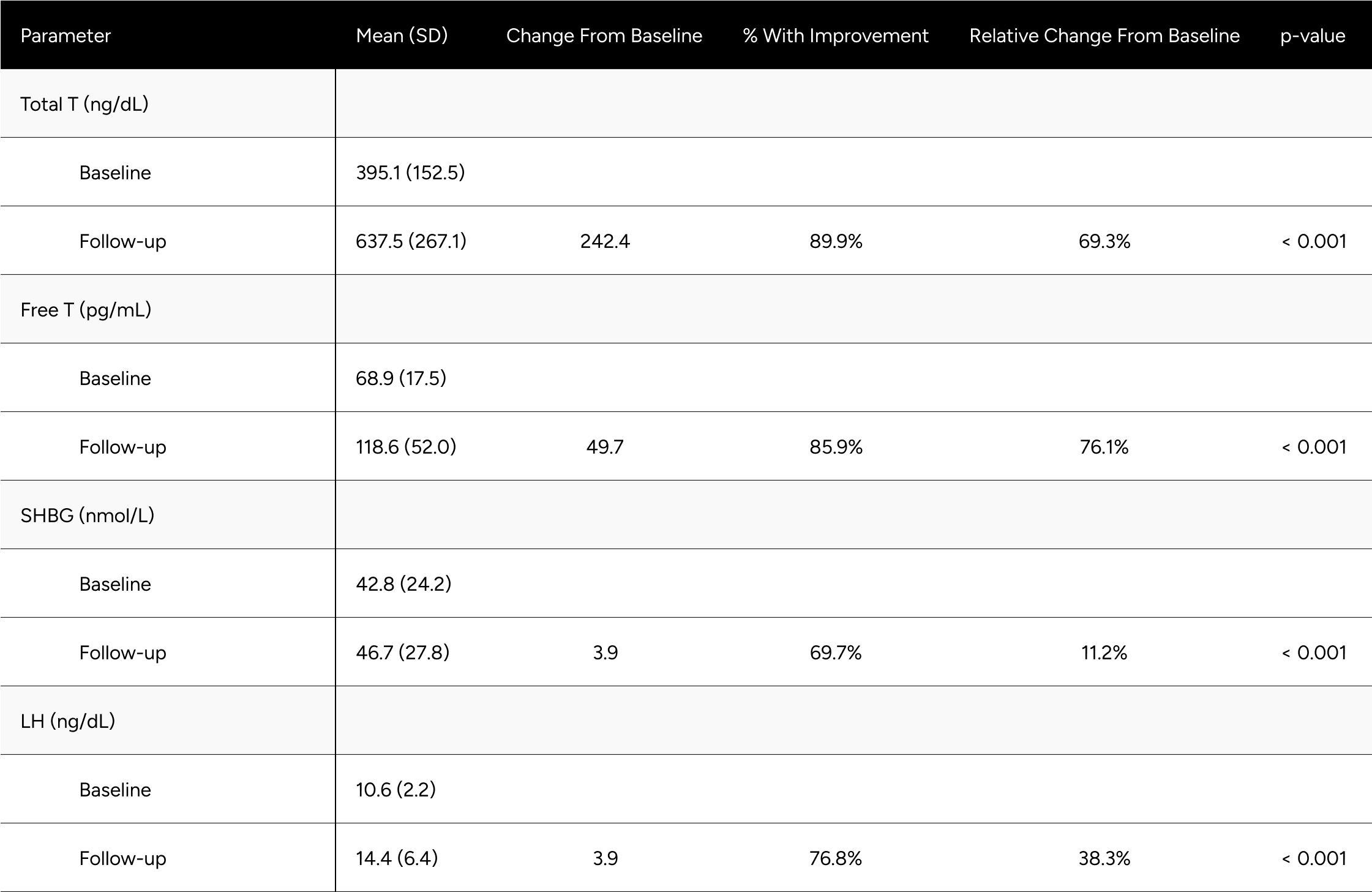
* Mixed Hypogonadal is defined as an LH above 8.7 and Free T between 65-97 pg/mL
Table A3: Average Changes in Hormones Between Baseline & Follow-up For Eugonadal* Men
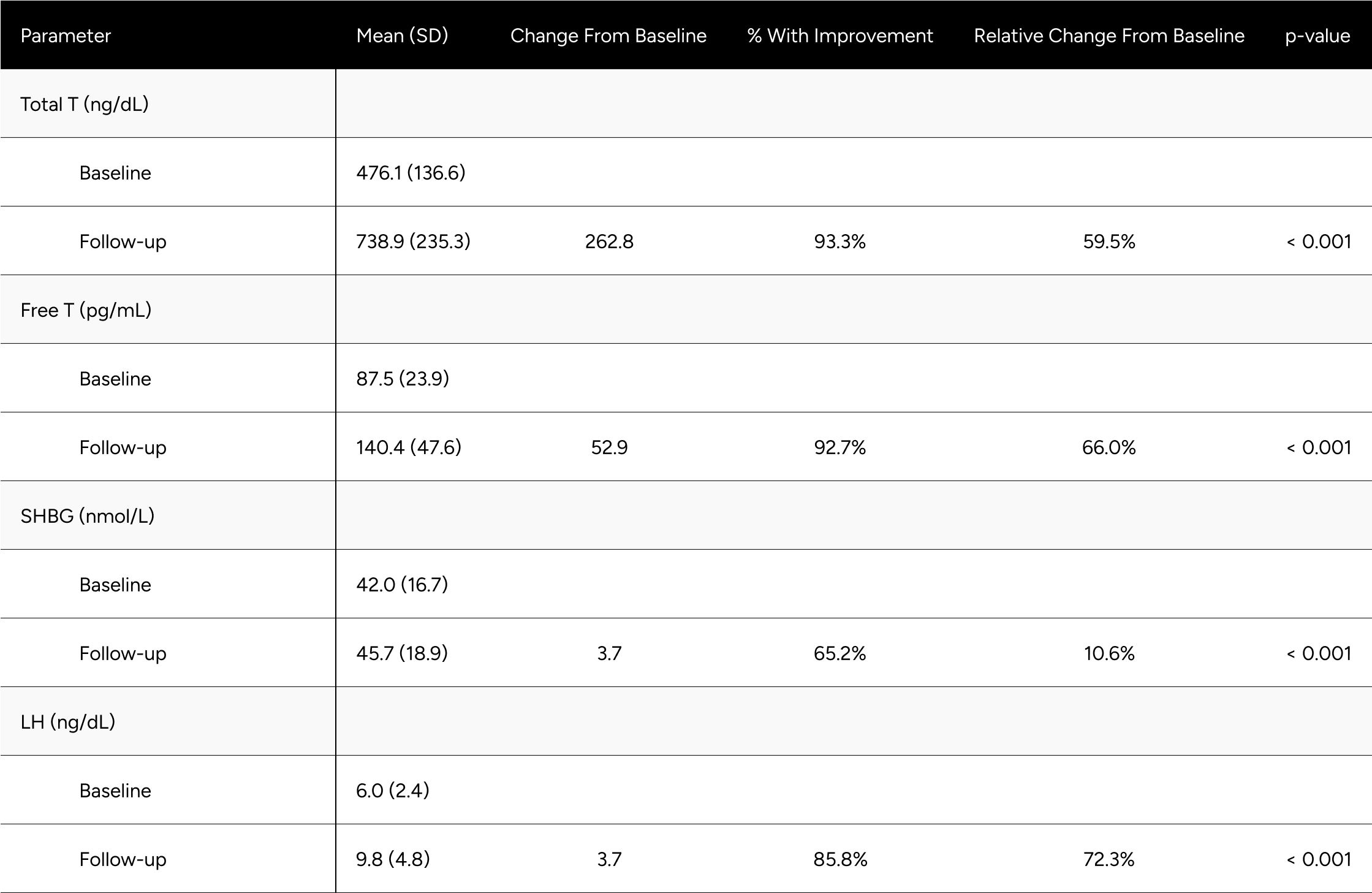
* Eugonadism is defined as a Total T >= 300 ng/dL.
Table A4: Overview of Treatment Emergent Adverse Events (TEAEs) Possibly Related to the Study Drug (N=80)
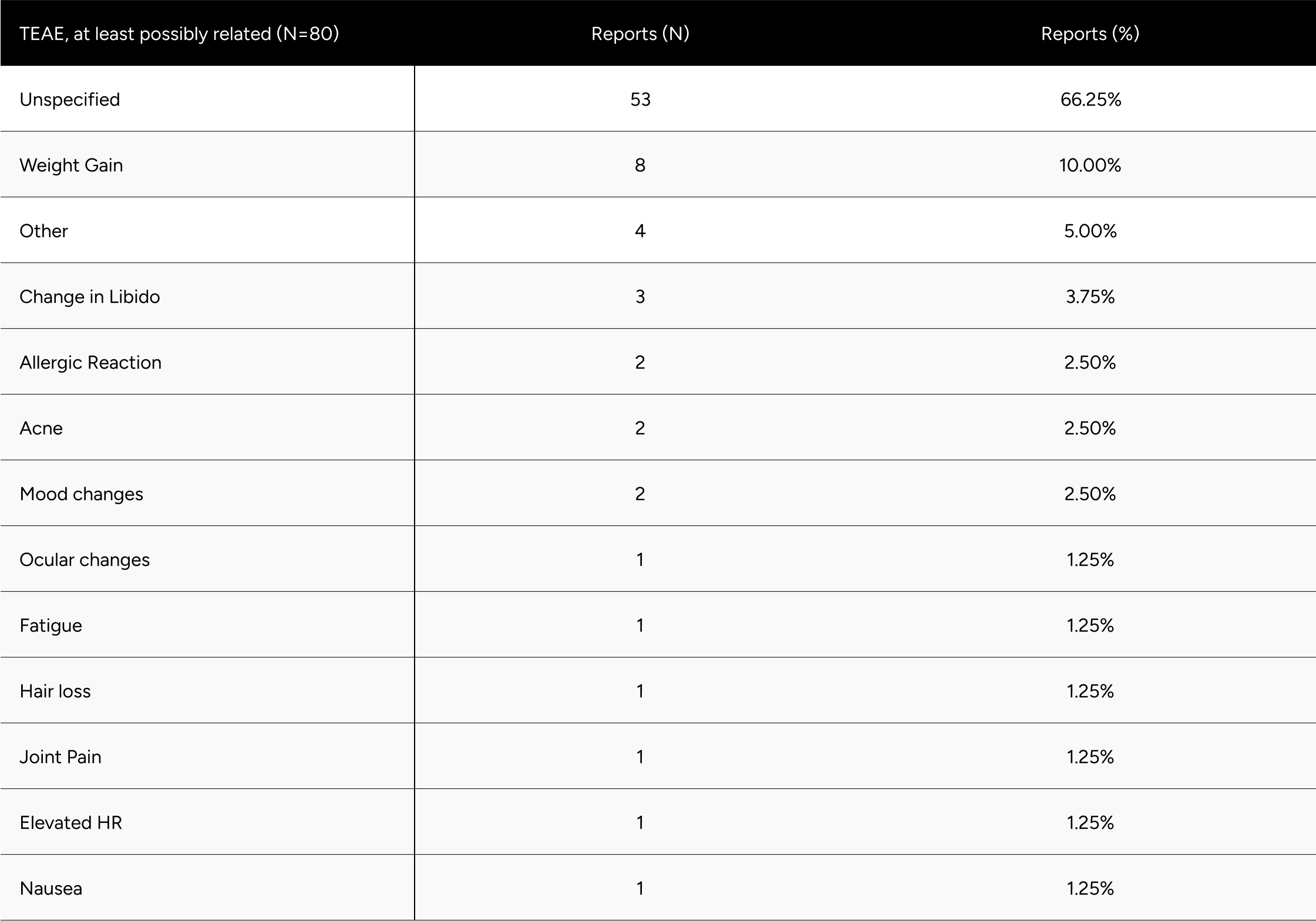
* Men without a decrease in Free-Testosterone.
* 80 participants who demonstrated a decline in testosterone levels during treatment .It is hypothesized that these participants had undiagnosed primary hypogonadism or did not accurately report their ongoing TRT regimen during their baseline test.
Supplementary Material
Qadam questionnaire
The qADAM responses generate a composite score ranging from 10-50, with a lower score being indicative of potential hypogonadism.22 Responses to each of the questions are collected on a five-point scale.
The qADAM questionnaire is as follows:
- How would you rate your energy level?
- How would you rate your strength/endurance?
- How would you rate your enjoyment of life?
- How would you rate your happiness level?
- How would you rate your libido (sex drive)?
- How strong are your erections?
- How would you rate your work performance over the last 4 weeks?
- How often do you fall asleep after dinner without intending to?
- How would you rate your sports ability over the last 4 weeks?
- How much height have you lost in your lifetime?
Response scoring: (exception of the question related to height loss)
- Terrible = 1
- Poor = 2
- Average = 3
- Good = 4
- Excellent = 5
Responses of 3 (Average), or higher were categorized as normal function. Total qADAM composite scores of 30 or higher (indicating an average response of 3 or higher per question) were categorized as normal function.
Baseline height loss responses are assumed constant for follow-up, given participants could not reasonably lose additional height within the time duration of the study.
The PHQ-4 is a brief screening questionnaire that assesses anxiety and depression.23 Participants are asked to rate the frequency of the following symptoms over the last two weeks, to the point of being bothered:
The anxiety & depression questions:
- Feeling nervous, anxious, or on edge
- Not being able to stop or control worrying
- Feeling down, depressed or hopeless
- Little interest or pleasure in doing things
Response scoring:
- Not at all = 0
- Several days = 1
- More than half the days = 2
- Nearly every day = 3
Response points are summed to determine overall function: normal (0-2), mild (3-5), moderate (6-8), and severe (9-12).
The responses to the first two questions are summed to assess anxiety, where a score >=3 suggests anxiety.
The responses to the second two questions are summed to assess depression, where a score >=3 suggests depression.











