Abstract
Men with low testosterone that have taken Enclomiphene Citrate have been shown to increase their total and free T levels by ~90%.
Findings:
- Total and free testosterone levels (85% and 90% relative increase at follow-up, respectively, p<0.001)
- Luteinizing hormone (96%, p<0.001)
- Sex hormone binding globulin (18%, p<0.001)
- qAdam and PHQ-4 scores (78.6% and 67.2% with improvement, respectively, p<0.001)
Declining Testosterone
Men’s testosterone tends to decrease with advancing age.[1]
These age-related decreases are noticeable among men in their 30s and 40s, even earlier for some. Men who are obese, have chronic diseases such as type II diabetes, poor sleep, and are fighting off infectious diseases tend to have lower testosterone.[2]
Other factors like stress, poor nutrition, sedentary lifestyle, lack of sun exposure, and exposure to microplastics also impact testosterone negatively in all ages. Because more men are living longer lives and are increasingly exposed to detrimental environments, we are witnessing more and more men with low testosterone at all ages.
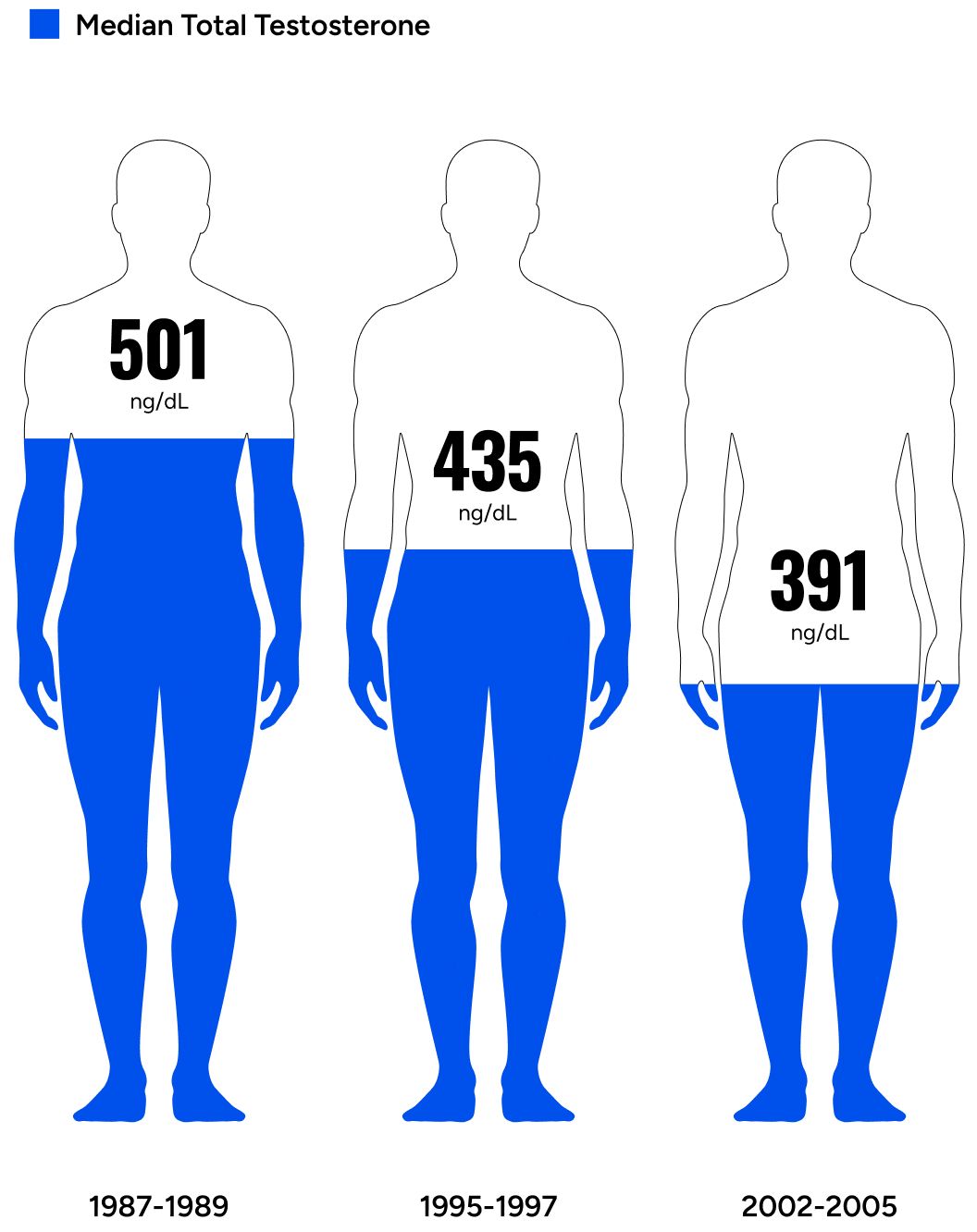
Men’s testosterone levels are decreasing across generations.
A landmark study looking at testosterone levels in men across generations concluded, “The past 20 yr have seen substantial age-independent decreases in male serum testosterone (T) concentrations, decreases that do not appear to be the consequence of the contemporaneous trends in health and lifestyle considered here.”
Men with low testosterone often experience lack of motivation, lack of energy, low libido, poor life outlook, fat accumulation, increased risk for chronic conditions, and countless other undesirable outcomes.
`The standard definition for low testosterone from the American Urology Association is 300 ng/dL.[3] However, defining low testosterone based solely on total testosterone is often misleading. Men may meet the standard definition of low testosterone but present no symptoms. Men who receive treatment may achieve “normal” levels of total testosterone and not feel symptoms improvement.[4] Treating men based only on their total testosterone levels may fail to address the complexity of hypogonadism.
As explained by Paduch et al, many assays used to define “normal” are developed in older men and are not necessarily representative of expected “normal” levels in younger, healthier men. They also argue that measurements of free testosterone or bioavailable testosterone should be used in evaluating patients, especially when total testosterone levels are “equivocal or fail to reflect clinical presentation”.[5] Said differently, men who meet standard eugonadal definitions may still present hypogonadal symptoms, highlighting the importance of taking patient symptoms into consideration along with lab validated biomarkers when evaluating and treating patients.
Options For Treating Hypogonadism
Testosterone replacement therapy (TRT) has been the default treatment for men with low t levels.[6]
There are many forms of TRT available. For example, a common form of TRT is administered through injecting it into your muscles. Other forms of TRT include patches (that you can stick to your skin) and gels (that you rub into your skin). TRT, as the name suggests, replaces deficient levels of testosterone with synthetic testosterone from an external source.
Some of the primary reasons men take TRT are to support libido and sexual activity, maintain muscle and decrease fat, maintain bone strength and function, and enhance energy and cognition. Impacts on motivation, muscle and strength are reasons athletes have used synthetic testosterone for performance enhancement.
Many men who want to discontinue standard TRT struggle to do so because of withdrawal.[7]
Stopping TRT can cause:
- weight gain
- low energy
- low sex drive
- irritability
- severe depression
Side Effects of trt:
- prostate cancer progression
- polycythemia
- peripheral edema
- cardiac and hepatic dysfunction
TRT is not the only treatment for men experiencing low testosterone.
Enclomiphene citrate, a selective estrogen receptor modulator (SERM), works by increasing testosterone levels.
It stimulates your body to produce its own testosterone. Enclomiphene citrate is taken orally. It does not contain synthetic testosterone. Scientists compared enclomiphene to 1.62% Androgel in overweight men aged 18-60 years with low-normal testosterone (≤300 ng/dL). Over 16 weeks, enclomiphene citrate consistently increased LH, FSH and testosterone. It also kept sperm concentrations in the normal range, while Androgel reduced sperm counts.[8]
A study reported, “Enclomiphene is a very promising drug for patients with secondary hypogonadism [low T due to poor signaling in the body] and who are concerned about the negative effects of exogenous testosterone . . . Ideally, this therapy could become the primary medication for men with secondary hypogonadism who wish to preserve spermatogenesis [the production of sperm].”[9]
Side effects
Potentially lower risk of estrogenic side effects: Zuclomiphene, the estrogenic isomer in clomiphene, can bind to estrogen receptors and may cause estrogenic side effects such as hot flashes, mood swings, and gynecomastia (enlargement of male breast tissue). Enclomiphene, being devoid of the estrogenic isomer, may potentially have a lower risk of estrogenic side effects.
Single-isomer
Enclomiphene is a single-isomer form: Clomiphene is a mixture of two isomers, enclomiphene and zuclomiphene, with the latter having estrogenic effects. Enclomiphene is the active isomer that specifically blocks estrogen receptors in the hypothalamus, resulting in increased release of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), which in turn stimulate testosterone production. Enclomiphene, being the single-isomer form, is thought to have a more targeted and predictable effect compared to the mixed isomers in clomiphene.
Half-life
Shorter half-life: Enclomiphene has a shorter half-life compared to clomiphene, which means it is cleared from the body more quickly. This may allow for more precise dosing and flexibility in adjusting treatment regimens.
Sperm quality
Potential for better sperm quality: Clomiphene is commonly used off-label for the treatment of male infertility, but it can negatively impact sperm quality in some men due to its estrogenic effects. Enclomiphene, being devoid of the estrogenic isomer, may potentially have a more favorable effect on sperm quality.
Other treatments for male hypogonadism not discussed in this paper include aromatase inhibitors (AI) and human chorionic gonadotropin (hCG).[10]
To date, research on enclomiphene has focused on men presenting secondary hypogonadism as defined by total testosterone levels.[11] The following study characterizes enclomiphene’s effectiveness at increasing testosterone and alleviating hypogonadal symptoms in a cohort of participants presenting diverse demographic and health statuses. Some participants met traditional hypogonadal definitions based on total testosterone at baseline, while others presented hypogonadal symptoms with “eugonadal” testosterone levels.
Enclomiphene citrate, a selective estrogen receptor modulator (SERM), works by increasing testosterone levels.
Hypotheses
At the onset of our research we set the following hypotheses:
1.
Enclomiphene achieves clinically significant increases in free testosterone, defined as a 50% increase at follow-up
2.
Enclomiphene significantly increases qAdam and PHQ-4 scores at follow-up
Patients and Methods
Study Population
All patients were men between 18 and 69 years of age, and presented symptoms of hypogonadism during a baseline questionnaire.
For inclusion in this study, participants must have completed a baseline lab test (pre-treatment), at least one follow-up lab test (post-treatment), a baseline medical questionnaire (pre-treatment), and a follow-up medical questionnaire (post-treatment). The follow-up lab test sample was necessarily collected after three to four weeks of treatment following dosage titration to ensure that peak serum medication levels were achieved.
Patients start on a dosage of 3.125mg, 6.25mg, or 12.5mg. Starting dosage is determined by the patient’s board-certified doctor based on their evaluation of baseline lab results and baseline questionnaire. After three to four weeks into treatment, the patient is evaluated for symptom alleviation and signs of adverse effects. Patients may be titrated up (as high as 25mg) or down in dosage, and asked to delay completion of their first follow-up lab kit until they have been treated for three to four weeks on their titrated dose. Additional lab testing may be required after subsequent dosage titration.

participant Exclusions:
- testosterone replacement therapy
- anabolic steroids
- selective androgen receptor modulators (SARMs)
- aromatase inhibitors (AIs)
- selective androgen receptor modulators (SERMs)
Other exclusion criteria were contraindications for treatment, including: known allergies to SERMs, a history of prostate cancer, blood thinner usage. All participants were necessarily 18 years or older and assigned male at birth.
Men presenting a Luteinizing Hormone (LH) less than 1.0 mIU/mL were excluded due to strong indication of one of the following:
1. Hypogonadotropic hypogonadism
This is a condition where there is inadequate production of LH and follicle-stimulating hormone (FSH) from the pituitary gland, leading to decreased testosterone production. It can be caused by various factors, such as dysfunction of the hypothalamus or pituitary gland, genetic disorders, tumors, certain medications, stress, or other underlying medical conditions.
2. Testicular failure
Conditions that affect the testicles, such as trauma, infection, radiation or chemotherapy, certain medications, or genetic disorders, can result in decreased production of testosterone and subsequently low LH levels.
3. Hormonal therapY
Some hormonal therapies, such as androgen deprivation therapy (ADT) used in the treatment of prostate cancer, can suppress LH levels as part of the treatment strategy to reduce testosterone production.
The patients included in the study were required to have completed a follow up lab test after completing at least 4 weeks medication on the King protocol.
807 men
between 18 and 69 years of age
Of the 807 men, 778 had an initial LH of 1 mIU/mL. Due to the influence of patients taking steroid influencing medications that would significantly influence the results of the study, men that were on any of the following medications during onboarding were excluded from the study, including Testosterone Injections (TRT), Anabolic Steroids, Clomid, Aromatase Inhibitors, or other Serms. Excluding 74 men that had specified their use of steroid inducing medications and another 128 that had not completed the full medical questionnaire at the point of onboarding, 576 patients remained.
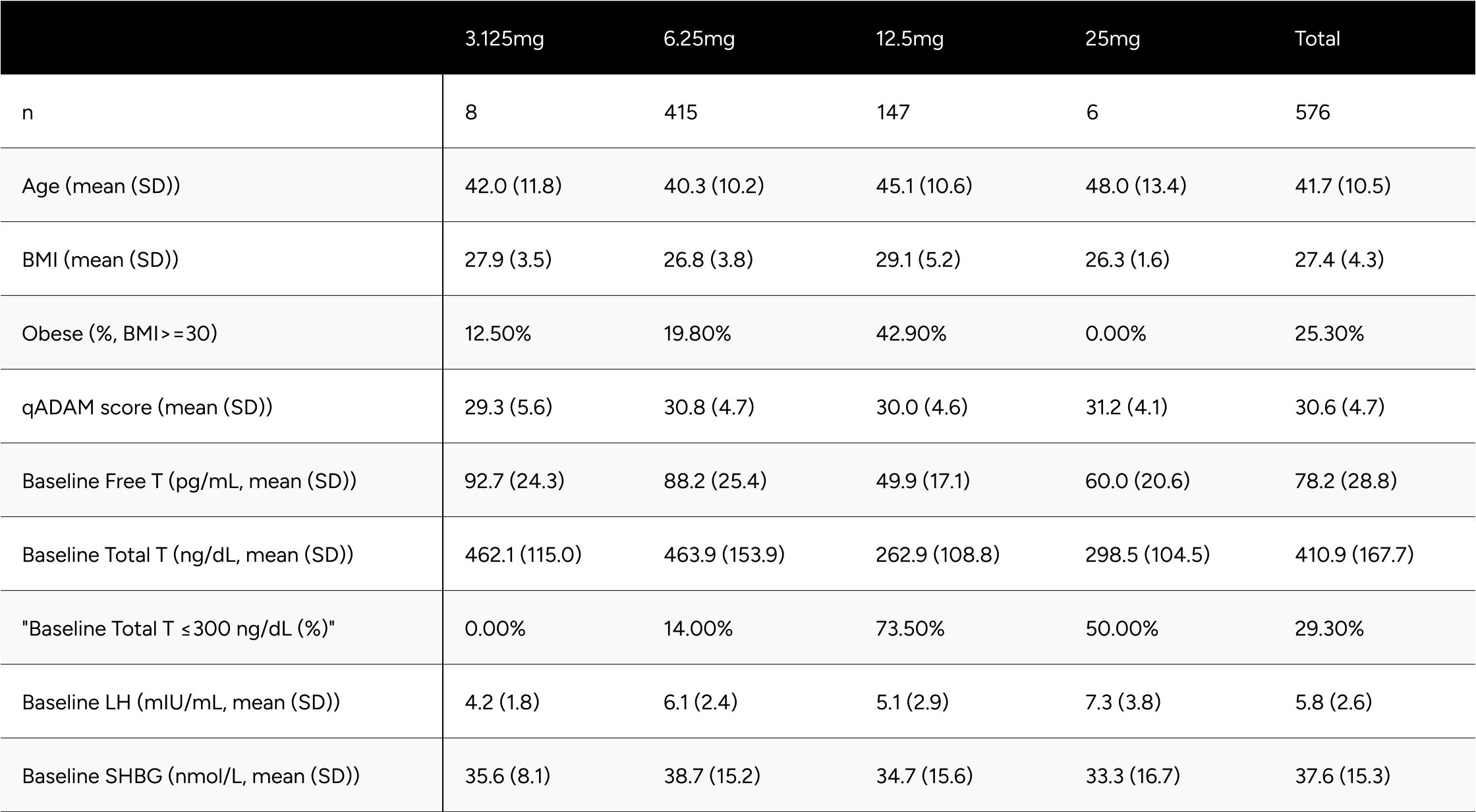
Table 1 provides descriptive statistics for age, self-reported BMI, qADAM score, and baseline biomarkers for the study population. These descriptive statistics are also broken out by the final dosage patients were at the time of their most recent follow-up lab test. Notably, only 8 and 6 patients were on 3.125mg and 25mg respectively. Compared to patients on 6.25mg, patients on 12.5mg were older (45.1 vs 40.3 years old), had a higher BMI (29.1 vs. 26.8 BMI), and had a lower baseline free testosterone (49.9 vs 88.2 pg/mL). Interestingly, qAdam scores were similar (30.0 vs 30.8).
Table 1: Study Population Descriptive Statistics
Data Collection
Patient demographics, health status (biomarker and self-reported), and treatment data are collected from on-line medical questionnaires, CLIA-certified at-home lab tests, and an electronic medical record system.
Medical Questionnaire
The medical questionnaire completed at baseline (pre-treatment) and follow-up (three to four weeks into treatment) are self-administered online by patients via the Maximus application.
The questionnaires cover a variety of domains, from lifestyle behaviors, chronic conditions, qADAM (Androgen Deficiency in Aging Males questionnaire), PHQ-4, height, weight, body fat, current medications, allergies, and condition history (self and family).
The qADAM questions are used to calculate a composite score whose values range from 10-50, where a lower score indicates potential hypogonadism.[12] Responses to each of the questions is collected on a five-point scale.
The individual qADAM questions:
- How would you rate your energy level?
- How would you rate your strength/endurance?
- How would you rate your enjoyment of life?
- How would you rate your happiness level?
- How would you rate your libido (sex drive)?
- How strong are your erections?
- How would you rate your work performance over the last 4 weeks?
- How often do you fall asleep after dinner without intending to?
- How would you rate your sports ability over the last 4 weeks?
- How much height have you lost in your lifetime?
response scoring:(exception of the question related to height loss)
- Terrible = 1
- Poor = 2
- Average = 3
- Good = 4
- Excellent = 5
Responses of 3 (Average), or higher were categorized as normal function. Total qADAM composite scores of 30 or higher (indicating an average response of 3 or higher per question) were categorized as normal function.
Baseline height loss responses are assumed constant for follow-up, given participants could not reasonably lose additional within the time duration of the study.
The PHQ-4 is a brief screening questionnaire that assesses anxiety and depression. [13] Questions are asked the rate the frequency of the following symptoms over the last two weeks, to the point of being bothered:
The anxiety & depression questions:
- Feeling nervous, anxious, or on edge
- Not being able to stop or control worrying
- Feeling down, depressed or hopeless
- Little interest or pleasure in doing things
response scoring:
- Not at all = 0
- Several days = 1
- More than half the days = 2
- Nearly every day = 3
Response points are summed to determine overall function: normal (0-2), mild (3-5), moderate (6-8), and severe (9-12).
The first two questions summed to assess anxiety, where a score >=3 suggests anxiety.
The second two questions summed to assess depression, where a score >=3 suggests depression.
All responses to the medical questionnaire are evaluated by the Maximus clinical staff to assess treatment eligibility and to titrate treatment dosage.
Lab Testing
Samples are processed by a CLIA-certified, high-complexity clinical laboratory. Panels may vary from patient to patient but necessarily include:
labs include:
- Total Testosterone (TT)
- Sex Hormone Binding Globulin (SHBG)
- Luteinizing Hormone (LH)
Free Testosterone (FT) is calculated using the Vermeulen equation using measured TT and SHBG, with assumed albumin of 4.3 g/dL.
Doctors request follow-up lab testing be completed typically 3-4 weeks after dosage titration to assess the impact of treatment on hormone levels.
Statistical Analysis
While the sample size was quite large, we tested the data for normality using the Shapiro-Wilk Test. With a null hypothesis of the data being normally distributed, there was sufficient evidence to reject the null hypothesis in favor of the data being not normally distributed. The lab results had a positive skew, thus we used the non-parametric Wilcoxon Signed-Rank test. In order to determine if the symptom improvement before and after treatment, we used the Chi-Squared Test of Independence.
In addition to the AUA’s standard definition for hypogonadism (Total Testosterone < 300 ng/dL), we benchmarked FT and TT levels against reference ranges representing healthy nonobese 19-40 year old men, as collected in the Framingham Heart Study.[14] This benchmark was evaluated as it represents target testosterone levels for men looking to feel and perform as they did in their youth and in good health.
Results
Biomarker Changes
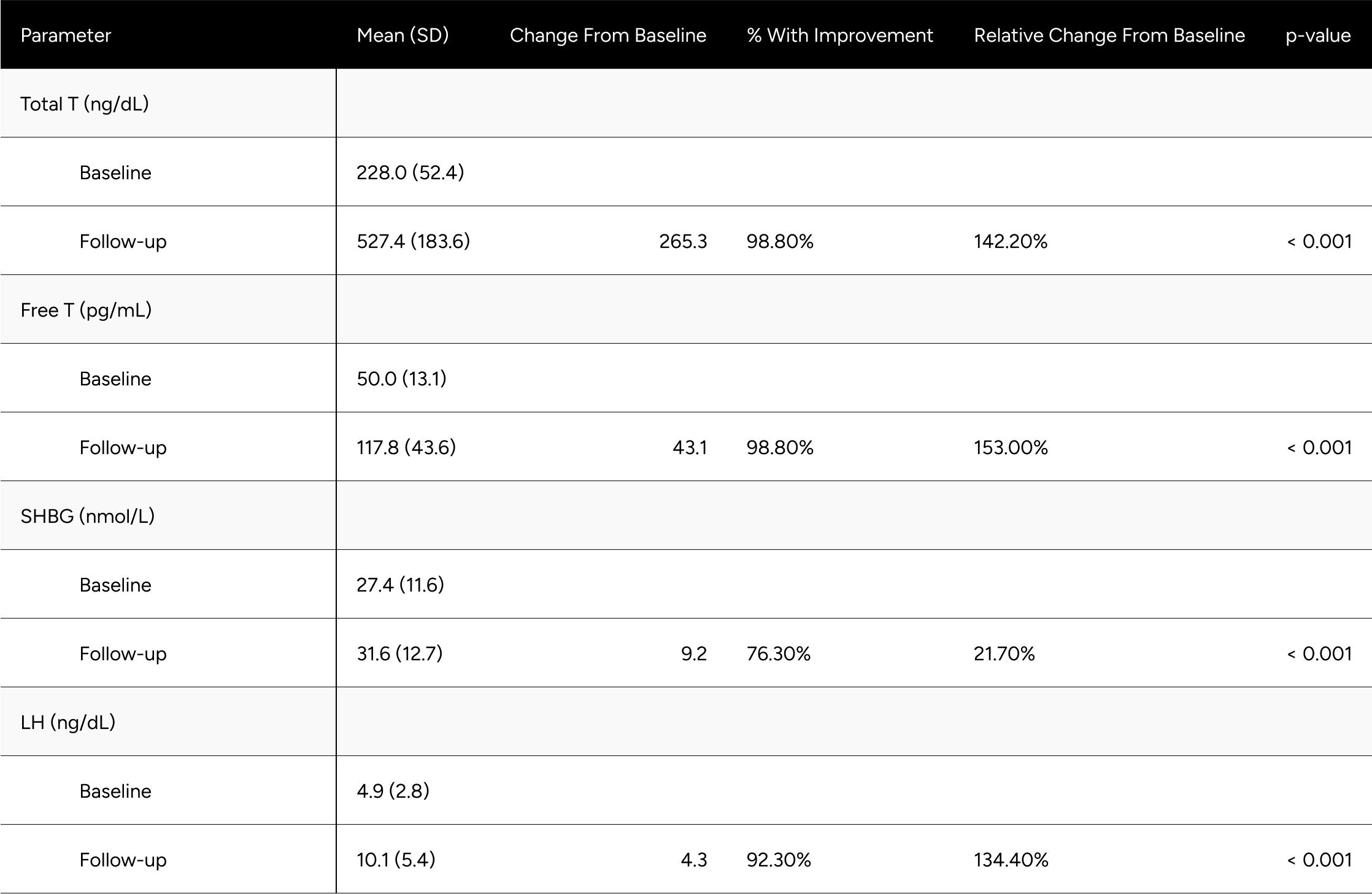
Treatment with enclomiphene significantly raised both TT and FT for study participants.
After treatment with enclomiphene:
57
Free Testosterone (ft)
FT increased 57.0 pg/mL on average (from 78.2 pg/mL to 135.2 pg/mL), which translates to a 90% increase on average for participants (p<0.001) (Table 2). At baseline, only 24.1% of participants were above -1 standard deviation of the average FT for healthy, non-obese 19-40 year olds. After treatment, 79.9% achieved this benchmark (increase of 55.8%). We observed 65.6% of participants achieved a 50% increase in FT from baseline (Table 3).
285
Total Testosterone (TT)
TT increased 285 ng/dL on average (from 410.9 ng/dL to 695.9 ng/dL), which translates to an 85% increase on average for participants (p<0.001) (Table 2). At baseline, only 27.4% of participants were above -1 standard deviation of the average TT for healthy, non-obese 19-40 year olds. After treatment, 78.6% achieved this benchmark (increase of 51.2%). At baseline, 70.7% of participants were above the standard eugonadal definition of 300 ng/dL TT, after treatment 97.6% (p<0.001). We observed 66.7% of participants achieved a 50% increase in TT from baseline (Table 3).
4.5
Luteinizing hormone (lh)
LH increased by 4.5 points on average, from 5.8 ng/dL to 10.5 ng/dL (p< 0.001). SHBG increased by 5.3 points, from 37.6 nmol/L to 42.9 nmol/L (p<0.001) (Table 2).
Despite significant increases in SHBG (18%), TT increased in such a manner (85%) that it “outpaced” SHBG and participants experienced disproportionate increases in FT (90%).
Table 2: Average Changes in Hormones Between Baseline & Follow-up
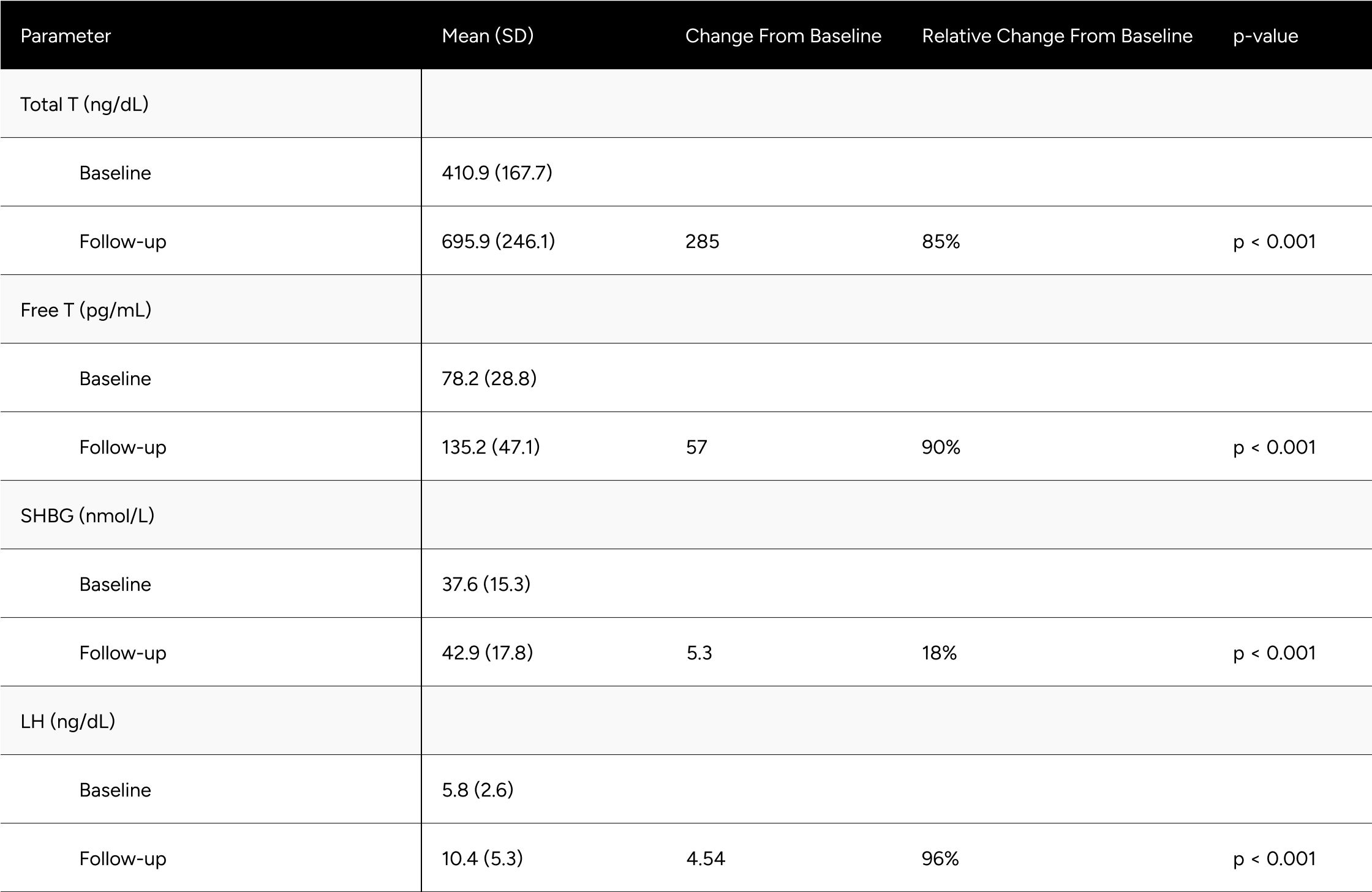
Table 3: Categorical Improvements in FT and TT
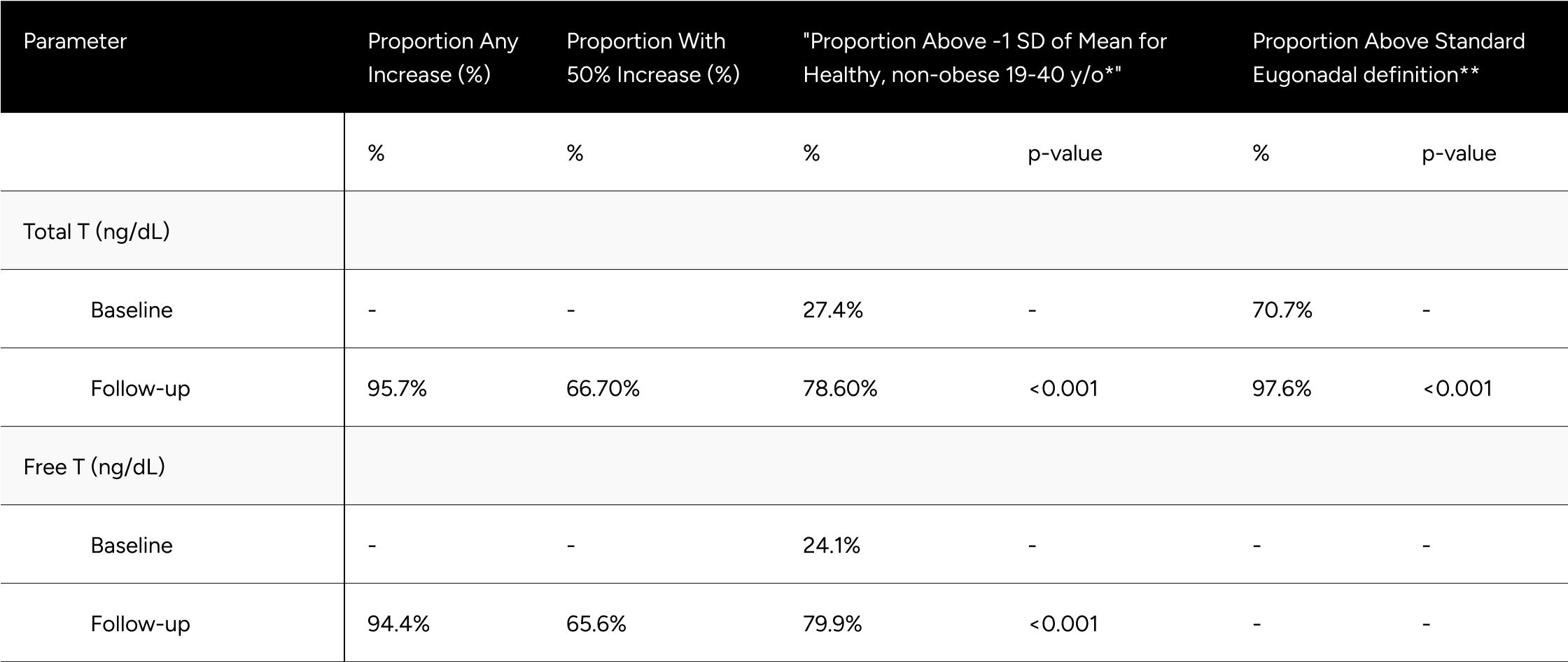
* Defined as 503 ng/dL for Total T, 97 pg/mL for Free T
** Defined as 300 ng/dL Total T. No definition set for Free T
We evaluated enclomiphene’s impact on hormone levels for “hypogonadal” and healthy/”eugonadal” participants independently separately. The definition for “hypogonadal” used was LH over 8.7 ng/dL and FT below 97 pg/mL at baseline.
Graphs and tables can be found in the Appendix.
Symptom Changes
Evaluating each qADAM question, questions with the lowest proportion of participants having normal function were energy (50.9%), sports ability (51.9%), and libido (49.3%). The question with the greatest proportion of participants having normal function was falling asleep after dinner (93.9%). The remaining questions, work performance, happiness, life enjoyment, erections, strength/endurance had normal function in 67.7%, 66.7%, 72.0%, 70.0%, and 72.9%, respectively.
Improvements at follow-up were seen at the highest rates for energy, sports ability, libido, happiness, life enjoyment, strength/endurance, with improvements 54.3%, 50.2%, 48.1%, 46.9%, 43.2%, 44.6%. Work performance, erections, falling asleep after dinner improved in 39.1%, 37.3%, 28.8%. Despite the variance in rate of improvement across the questions, all were statistically significant (p
After treatment, all qADAM questions showed the proportion of patients with normal function at or above 80% aside from energy (79.9%) and libido (72.0%). The proportion of patients with normal function was statistically significantly higher for all questions at follow-up (p
The composite qADAM score improved in 78.6% of patients (p
Table 4: Improvements in qADAM Scores
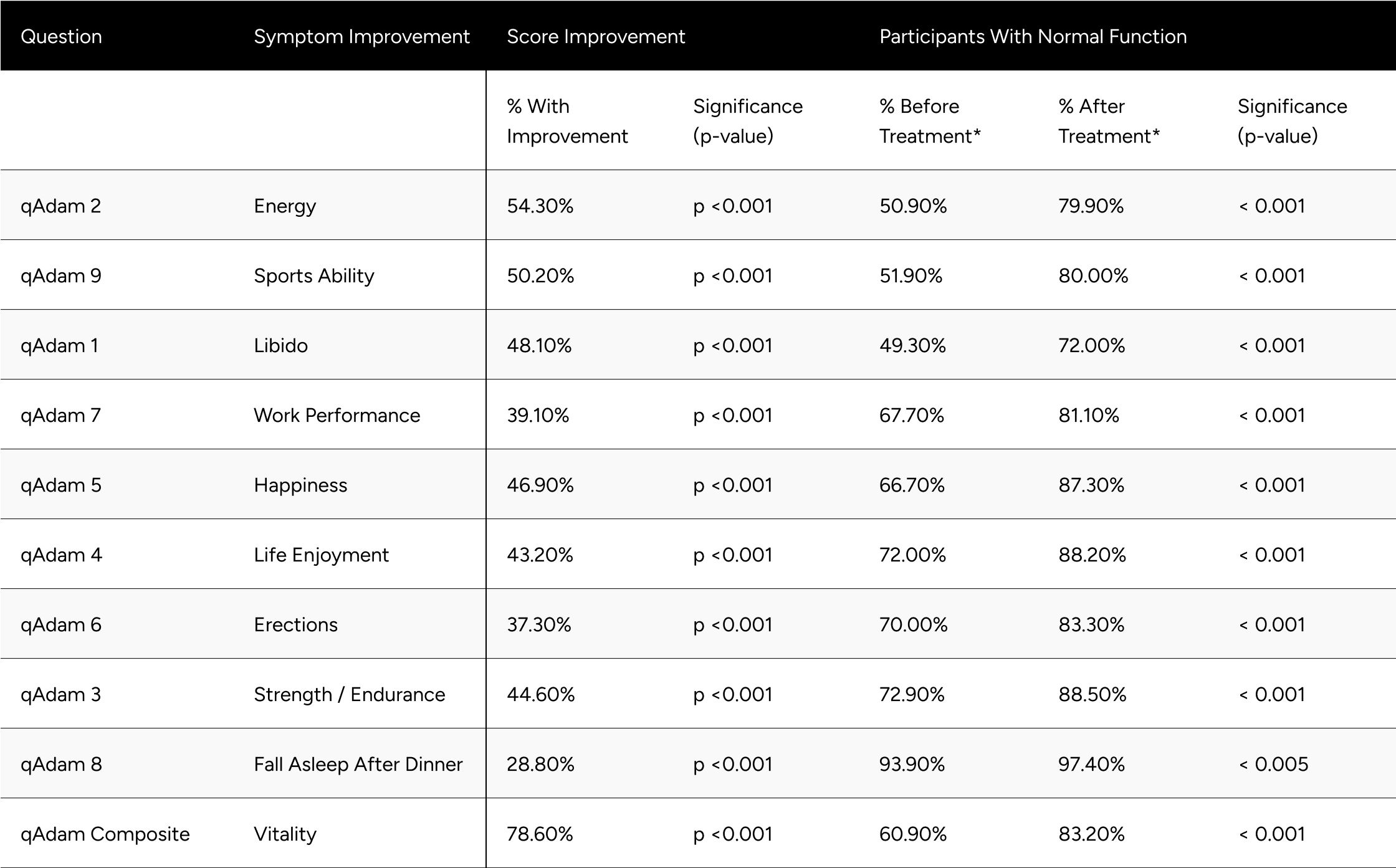
* Normal function is defined as a score of at least 3 for individual questions and average of 30 for the composite
Evaluating PHQ-4 components, we observed that 53.6% of respondents showed improvement in the anxiety score (p
Normal function (combined components) doubled, going from 26.4% at baseline to 53.8% of patients at follow-up (p
Table 5: Improvements in PHQ-4 Scores
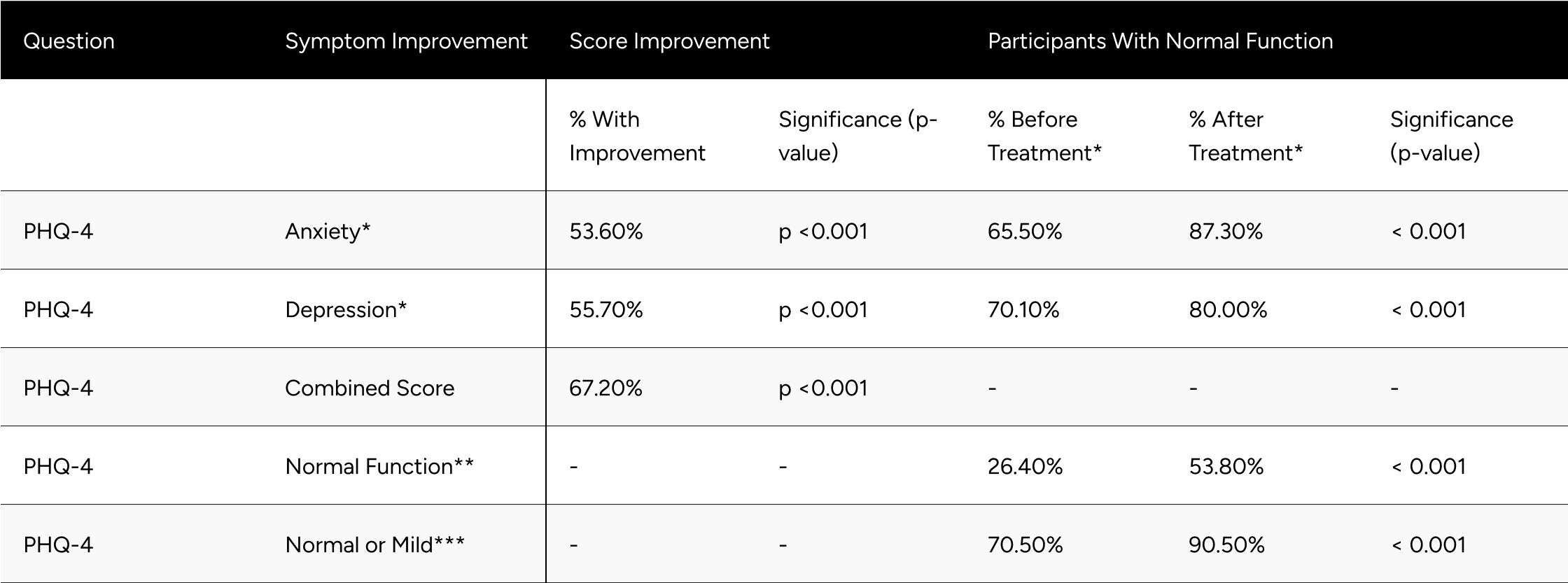
* Normal function PHQ-4 Anxiety and Depression is a score of less than 3.
** Normal function for the PHQ-4 combined score is a score of less than 3.
** Normal or mild function for PHQ-4 combined score is a score of less than 6.
Discussion
Findings
Hypogonadal and eugonadal men both respond overwhelmingly positively to treatment with enclomiphene, both in regards to hormonal improvements and self-reported symptom alleviation. Hypogonadal and eugonadal men both respond overwhelmingly positively to treatment with enclomiphene, both in regards to hormonal improvements and self-reported symptom alleviation.
Following treatment with enclomiphene, nearly every participant in the study surpassed the American Urology Association eugonadal threshold of 300 ng/dL TT. However, a TT of 300 ng/dL is between the 1st and 2.5th percentile for healthy, non-obese men aged 19-40 years olds. It is reasonable to conjecture that the vast majority of men would aspire to have the testosterone levels of a healthy, young man. As such, in our practice we also evaluate men against benchmarks set on that population.
Only 27.5% of participants were within one standard deviation of the mean TT for healthy, young men at baseline. Comparatively, 70.7% of participants met the 300 ng/dL threshold presented by the AUA at baseline. Considering that participants in this study were seeking treatment for hypogonadal symptoms, we believe the threshold set by the AUA may be too conservative and should be used in conjunction with patient symptoms and other benchmarks.
Treatment with enclomiphene successfully got 78.6% of participants within one standard deviation of the mean TT for healthy, young men at baseline. This nearly tripled the proportion at baseline, 27.4%.
As important, if not more so, is that participants experienced symptom alleviation.
Every qAdam question (besides loss of height) saw significant improvements in the study population. These questions were nearly all at 80% or higher “normal” function after treatment. The question that saw the proportion “normal” after treatment was libido, which has 72.0% of participants with normal function. Libido is complex and multifactorial.[15] Nevertheless, 48.1% of participants experienced improvements and the proportion with normal function increased from 49.3% to 72.0%. Future research may explore modulators of enclomiphene’s ability to improve libido, especially given its mechanism modulating estrogen, which is essential for libido in men.[16]
This study demonstrates that enclomiphene is a reasonable, if not preferred, treatment option for men experiencing symptoms of hypogonadism. Compared to traditional TRT, enclomiphene is convenient and, as validated in this study, non-suppressive of luteinizing hormone.
This study also demonstrates that men that do not meet the traditional definition of hypogonadism may benefit from treatment.
Limitations
Given participants were self-selected into treatment they paid for out of pocket, there may be selection bias in the study population towards men who responded favorably and thereby remained patients long enough to complete at least one follow-up lab. Hypogonadal and eugonadal men both respond overwhelmingly positively to treatment with enclomiphene, both in regards to hormonal improvements and self-reported symptom alleviation.
References
Appendix
Figure A1
Initial LH Influence on Free-T Response (%)
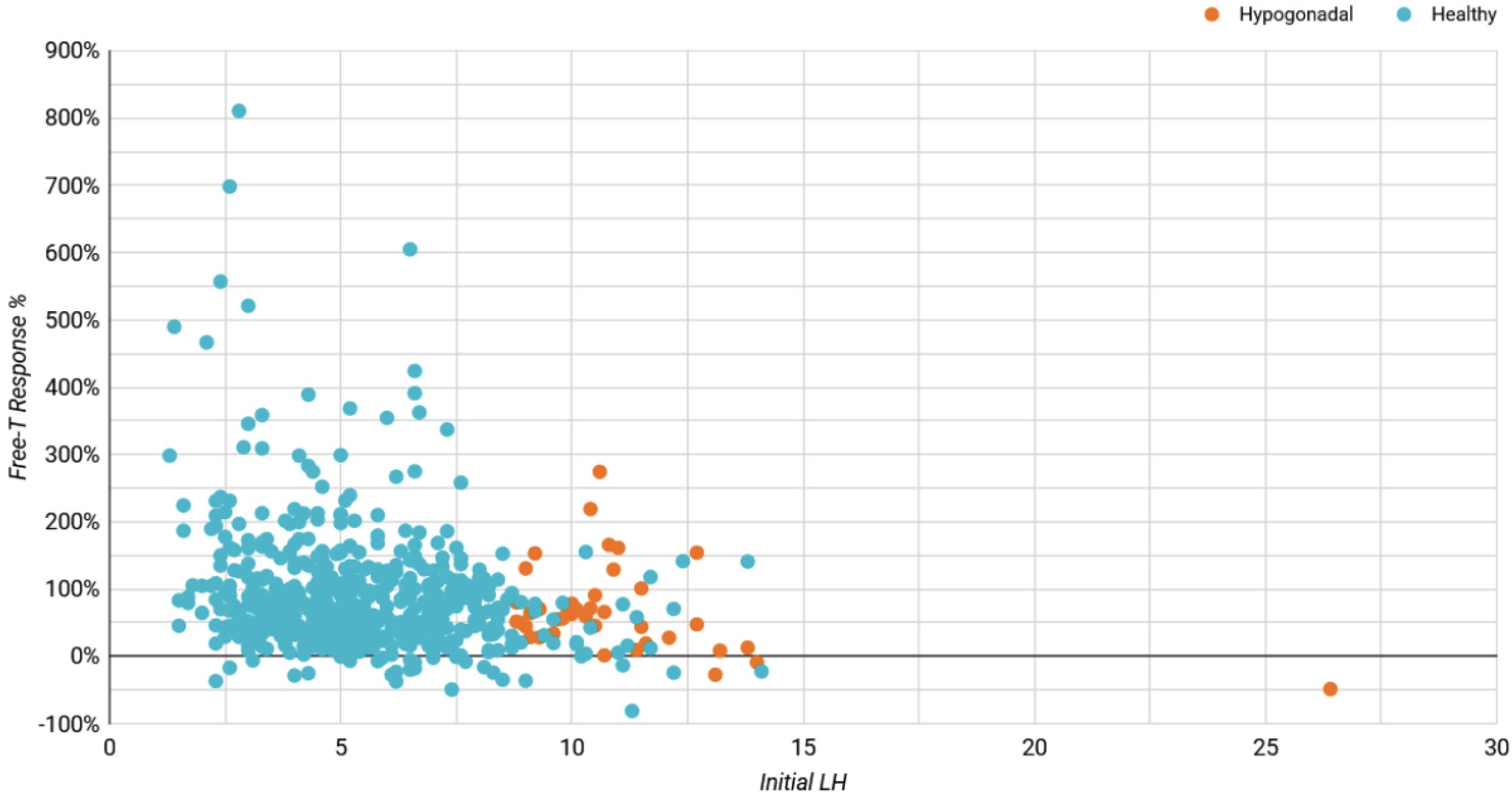
Table A1: Average Changes in Hormones Between Baseline & Follow-up For Hypogonadal Men
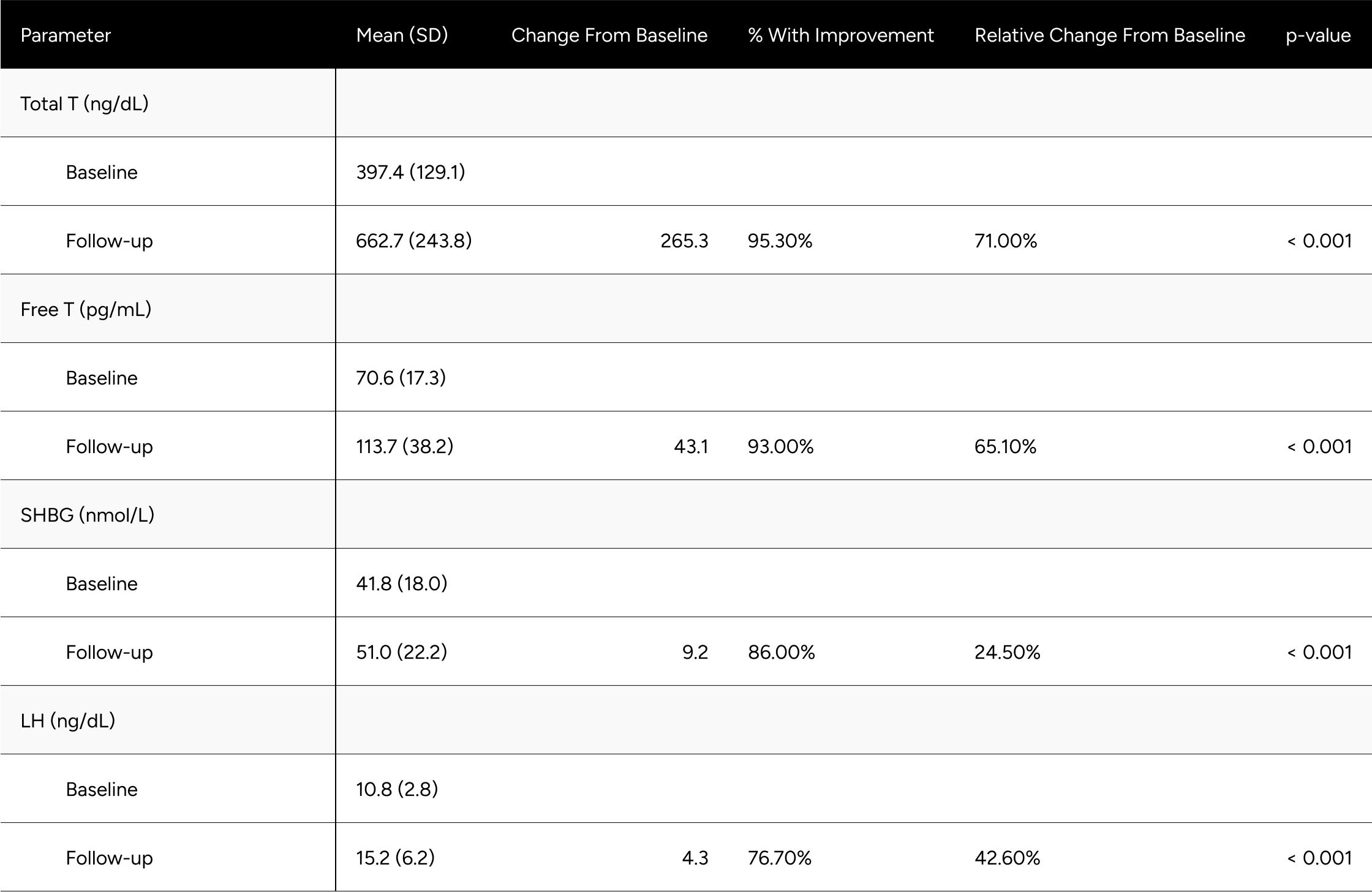
Table A2: Average Changes in Hormones Between Baseline & Follow-up For Eugonadal Men